JARDIANCE has proven to help patients across multiple indications
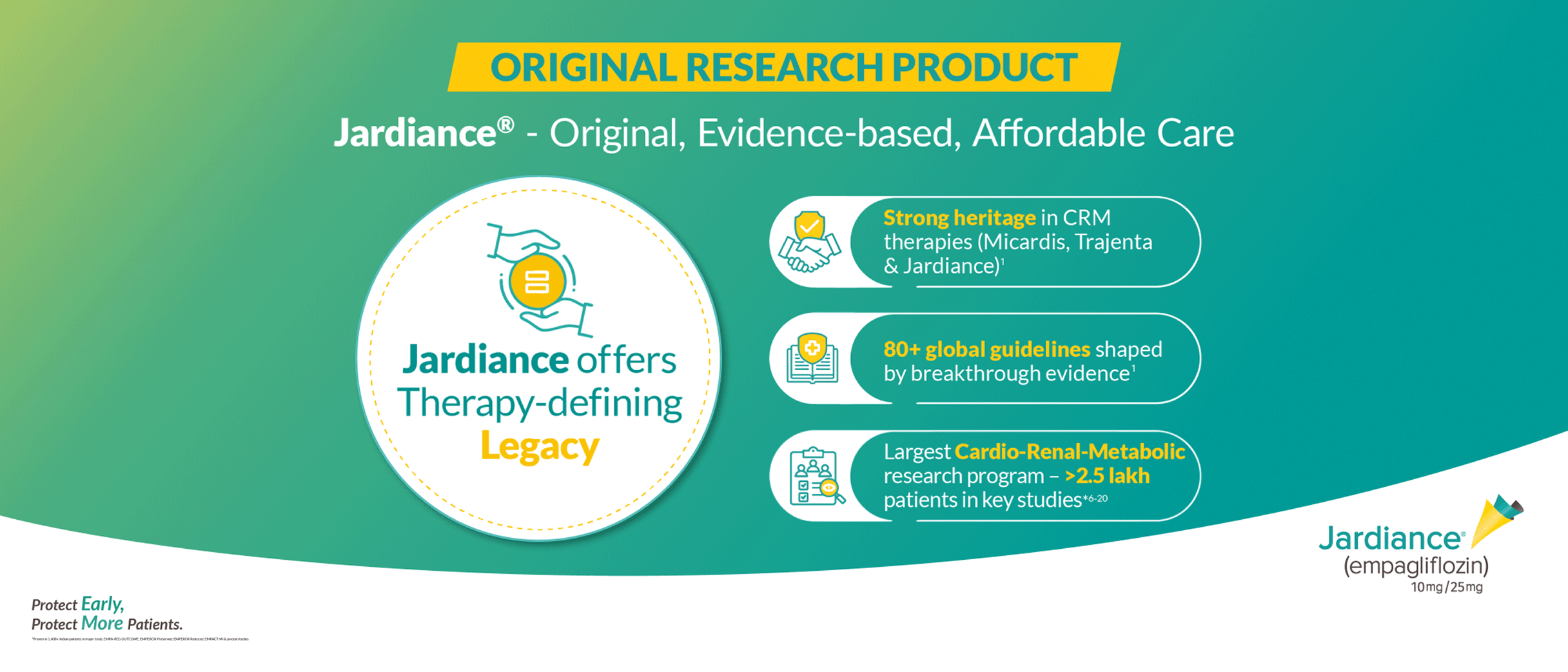
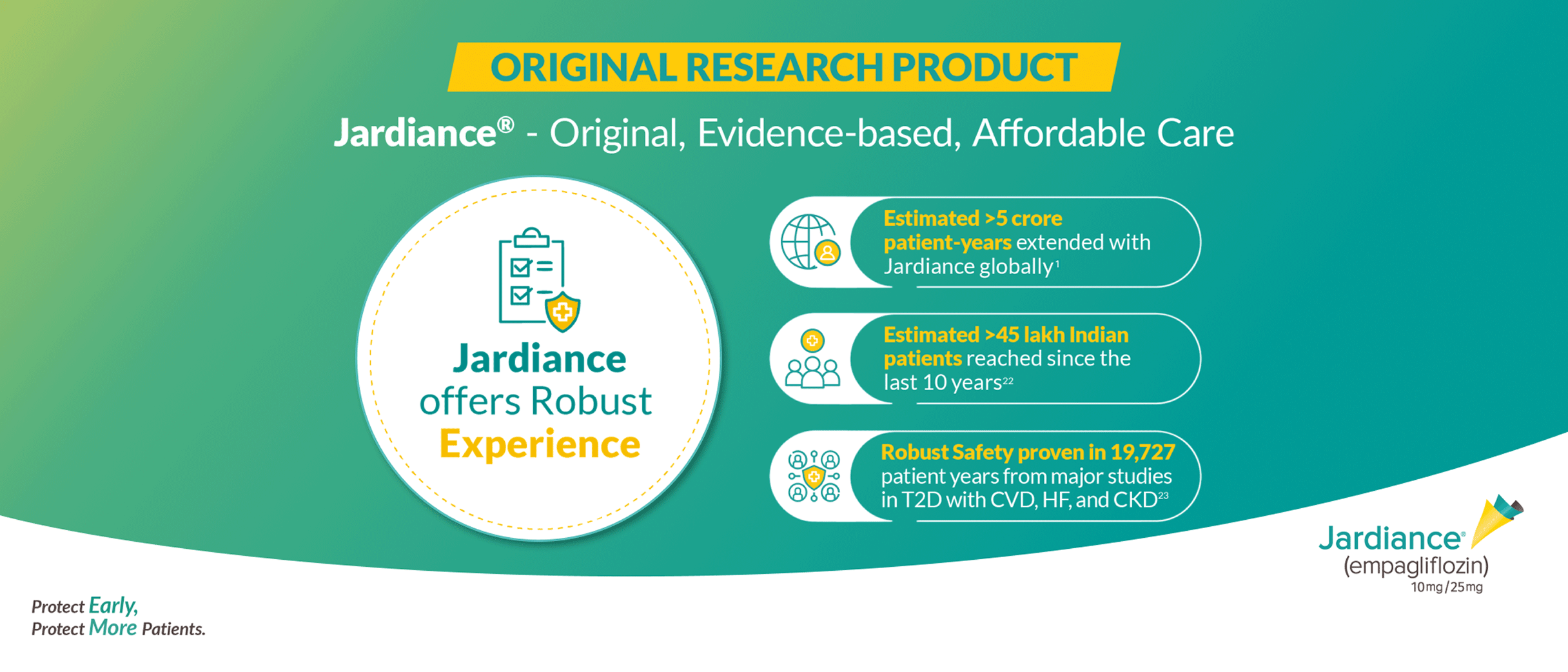
Built upon an established body of clinical evidence

Empagliflozin

Empagliflozin
-
†
Adjusted mean changes of –0.1% from baseline HbA1c 7.9% for placebo (n=207), –0.7% from baseline HbA1c 7.9% for JARDIANCE 10 mg (n=217), and –0.8% from baseline HbA1c 7.9% for JARDIANCE 25 mg (n=213), respectively. Difference from placebo (adjusted mean) was –0.6% for both JARDIANCE 10 mg and 25 mg; p<0.001 vs placebo for both doses.1,2
-
‡
In a subgroup analysis at 24 weeks of patients with baseline ≥ 8.5%, adjusted mean changes in HbA1c were –0.5% for placebo (n=50), –1.2% for JARDIANCE 10 mg (n=57), and –1.5% for JARDIANCE 25 mg (n=48). Difference from placebo (adjusted mean) was –0.73% for JARDIANCE 10 mg and –1.0% for JARDIANCE 25 mg. Missing data were imputed using the last observation carried forward (LOCF) approach.3
-
§
Adjusted mean change in systolic blood pressure from baseline at 24 weeks: JARDIANCE 10 mg –4.5 mmHg (n=217), JARDIANCE 25 mg –5.2 mmHg (n=213), vs placebo –0.4 mmHg (n=207).2
-
||
Adjusted mean changes of –0.45 kg reduction in body weight from baseline 79.7 kg for placebo (n=207), –2.08 kg from baseline 81.6 kg for JARDIANCE 10 mg (n=217), and –2.46 kg from baseline 82.2 kg for JARDIANCE 25 mg (n=213), respectively; p<0.0001 vs placebo for both doses.1,2
-
¶
In a subgroup analysis at 24 weeks of patients with baseline BMI ≥ 35, adjusted mean changes in weight were –0.34 kg for placebo (n=29), –2.63 kg for JARDIANCE 10 mg (n=33), and –3.35 kg for JARDIANCE 25 mg (n=41). Difference from placebo (adjusted mean) was –2.28 for JARDIANCE 10 mg and –3.01 for JARDIANCE 25 mg.3
-
#
In a double-blind extension trial, adjusted mean changes in weight for patients with baseline BMI ≥ 35 at Week 76 were 0.23 kg for placebo (n=29), –3.74 kg for JARDIANCE 10 mg (n=33), and –4.77 kg for JARDIANCE 25 mg (n=41). Difference from placebo (adjusted mean) was–3.96 kg for JARDIANCE 10 mg and –4.99 kg for JARDIANCE 25 mg. Missing data were imputed using the LOCF approach.3
-
&
The primary composite outcome in the EMPA-REG OUTCOME® trial was 3-point MACE, composed of death from CV causes, nonfatal MI, or nonfatal stroke, as analysed in the pooled JARDIANCE group vs the placebo group. Patients were adults with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke. The 14% RRR in 3-point MACE (HR=0.86; 95% CI: 0.74, 0.99; p<0.001 for noninferiority; p=0.04 for superiority) was driven by a reduction in the risk of CV death (HR=0.62; 95% CI: 0.49, 0.77); there was no change in risk of nonfatal MI (HR=0.87; 95% CI: 0.70, 1.09) or nonfatal stroke (HR=1.24; 95% CI: 0.92, 1.67).1,2
-
*
In the EMPEROR-Reduced trial, a randomised, double-blind, parallel-group, placebo-controlled study of 3730 patients with HFrEF, the efficacy and safety of JARDIANCE 10 mg (n=1863) were evaluated vs placebo (n=1867). Patients were adults with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤ 40%). The primary endpoint in the EMPEROR-Reduced trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE experienced a 25% RRR in this endpoint (HR=0.75; 95% CI: 0.65, 0.86; p<0.001).3
-
^
In the EMPEROR-Preserved trial, a randomised, double-blind, parallel-group, placebo-controlled study of 5988 patients with HFpEF, the efficacy and safety of JARDIANCE 10 mg (n=2997) were evaluated vs placebo (n=2991). Patients were adults with chronic heart failure (NYHA class II, III, or IV) and preserved ejection fraction (LVEF > 40%). The primary endpoint in the EMPEROR-Preserved trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE experienced a 21% RRR in this endpoint (HR=0.79; 95% CI: 0.69, 0.90; p<0.001).4
-
**
In the EMPA-KIDNEY trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6609 patients with CKD, the efficacy and safety of JARDIANCE 10 mg (n=3304) were evaluated vs placebo (n=3305). The primary endpoint in the EMPA-KIDNEY trial was a composite of CV death or progression of kidney disease. Patients treated with JARDIANCE experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64, 0.82; p<0.001).5
-
AKI, acute kidney injury; ARF, acute renal failure; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; CRM, cardio-renal-metabolic; CV, cardiovascular; CVD, cardiovascular disease; GMP, good manufacturing practices; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnea; RRR, relative risk reduction; SBP, systolic blood pressure; SBTi, science based targets initiative; T2D, type 2 diabetes.
-
Data on File, Boehringer Ingelheim.
-
Ved JK, et al. Presented at 6th International Diabetes Summit. Organized by Chellaram Diabetes Institute (virtual). 2022, March 4-6.
-
Data on File, https://www.boehringer-ingelheim.com/about-us/who-we-are/quality, Accessed 25th Feb 2025.
-
Data on File, https://www.boehringer-ingelheim.com/in/about-us/sites-around-world, Accessed 25th Feb 2025.
-
Data on File, https://www.boehringer-ingelheim.com/about-us/sustainable-development/more-green/boehringerreceives-sbti-validation-co2-reduction targets, Accessed 25th Feb 2025.
-
ClinicalTrials.gov. NCT03057977.
-
Packer M, et al. Eur J Heart Fail. 2019;21:1270.
-
Clinicaltrials.gov. NCT03057951.
-
Anker SD, et al. Eur J Heart Fail. 2019;21:1279.
-
ClinicalTrials.gov. NCT03448419.
-
Abraham WT, et al. Eur J Heart Fail. 2019;21:932.
-
ClinicalTrials.gov. NCT03448406.
-
ClinicalTrials.gov. NCT04157751.
-
Boehringer Ingelheim Pharmaceuticals, Inc. Press release. 2020. https://www.boehringer-ingelheim.com/press-release/dcri-collaboration-empact-mi.
-
Clinicaltrials.gov. NCT03332212.
-
ClinicalTrials.gov. NCT03594110.
-
Zinman B, et al. N Engl J Med. 2015;373:2117.
-
ClinicalTrials.gov. NCT03363464.
-
ClinicalTrials.gov. NCT03817463.
-
Patorno E, et al. Circulation. 2019;139:2822.
-
The Galien Foundation, https://www.galienfoundation.org/laureates-worldwide, Accessed 22nd Feb 2025.
-
Data on file, Boehringer Ingelheim. Estimated from IQVIA MAT 2024 data on sales, with assumption of treatment adherence as 3 months.
-
Wanner C, et al. Adv Ther. 2024 Jul;41(7):2826-44.
Vault ID: PC-IN-104667
Expiry date: 04/08/2027