JARDIANCE® protects by reducing risk for adult patients with1:
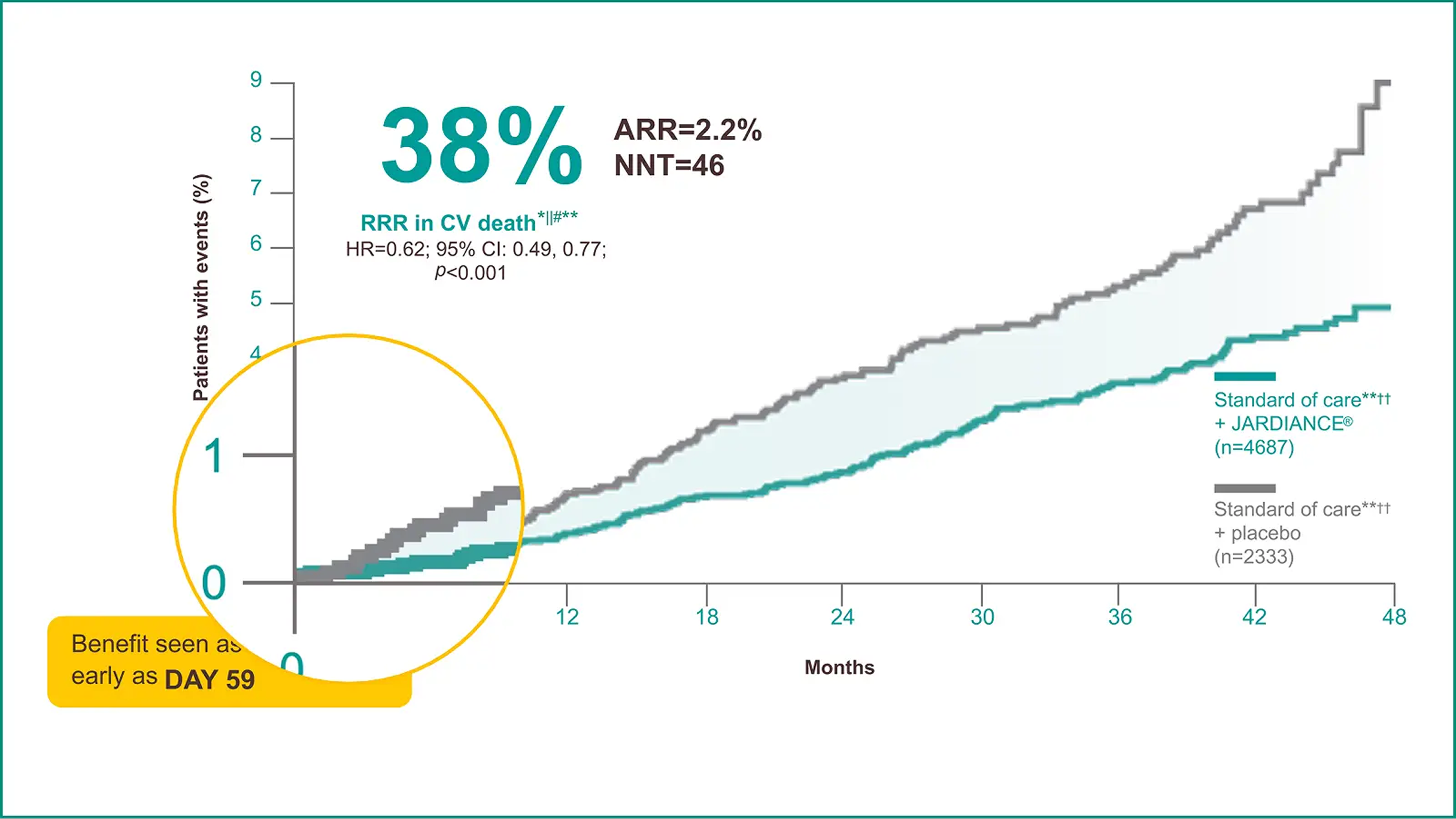
T2D + eCVD — reduced risk of CV death*1,2
CKD — reduced risk of CV death or kidney disease progression†1,3
HF — reduced risk of CV death or hospitalisation for HF‡§1,4,5

Type-2 diabetes significantly increases risk of untimely cardiovascular death
People with diabetes have 3 to 4 times higher risk of cardiovascular death.1,6
38%
(T2D + eCVD)*2
28%
progression;
NNT=28 (CKD)†3
25%
21%
38%
JARDIANCE® should be used first line in appropriate patients with T2D, CKD, or HF7-11
For your patients with T2D and eCVD||
- JARDIANCE® reduced the risk of CV death independent of multiple baseline characteristics‡‡§§2,19
- JARDIANCE® demonstrated results that can help extend life vs placebo||||20
- JARDIANCE® reduced the need for insulin21
Results from the EMPA-REG OUTCOME® trial.
For your patients with T2D and CV disease*





-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
-
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix.)
-
Herrington WG, Staplin N, Wanner C, et al; EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix.)
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. (EMPEROR-Preserved results and the publication’s Supplementary Appendix.)
-
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786.
-
McDonagh TA, Metra M, Adamo M, et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
-
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421.
-
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-733.
-
Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100.
-
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(suppl 4S):S117-S314.
-
Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes: implications for clinical practice. Prim Care. 1999;26(4):771-789.
-
UK Prospective Diabetes Study Group. UK prospective diabetes study 16. Overview of 6 years' therapy of type 2 diabetes: a progressive disease. Diabetes. 1995;44(11):1249-1258.
-
Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms. Diabetes Care. 2010;33(2):442-449.
-
Ma C-X, Ma X-N, Guan C-H, Li Y-D, Mauricio D, Fu S-B. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21(74):1-15.
-
American Diabetes Association. Cardiovascular disease. Accessed September 25, 2024. https://diabetes.org/about-diabetes/complications/cardiovascular-disease
-
Data on file. Boehringer Ingelheim Pharmaceuticals, Inc.
-
de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075-3090.
-
Inzucchi SE, Kosiborod M, Fitchett D, et al. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation. 2018;138(17):1904-1907.
-
Claggett B, Lachin JM, Hantel S, et al. Long-term benefit of empagliflozin on life expectancy in patients with type 2 diabetes mellitus and established cardiovascular disease: survival estimates from the EMPA-REG OUTCOME trial. Circulation. 2018;138(15):1599-1601.
-
Vaduganathan M, Inzucchi SE, Sattar N, et al. Effects of empagliflozin on insulin initiation or intensification in patients with type 2 diabetes and cardiovascular disease: findings from the EMPA-REG OUTCOME trial. Diabetes Obes Metab. 2021;23(12):2775-2784.
-
Verma S, Leiter LA, Sharma A, et al. How early after treatment initiation are the CV benefits of empagliflozin apparent? A post hoc analysis of EMPA-REG OUTCOME. Diabetes. 2020;69(suppl 1):28-OR.
-
*
The primary composite outcome in the EMPA-REG OUTCOME® trial was 3-point MACE, composed of death from CV causes, nonfatal MI, or nonfatal stroke, as analysed in the pooled JARDIANCE® group vs the placebo group. Patients were adults with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke. The 14% RRR in 3-point MACE (HR=0.86; 95% CI: 0.74, 0.99; p<0.001 for noninferiority; p=0.04 for superiority) was driven by a reduction in the risk of CV death (HR=0.62; 95% CI: 0.49, 0.77); there was no change in risk of nonfatal MI (HR=0.87; 95% CI: 0.70, 1.09) or nonfatal stroke (HR=1.24; 95% CI: 0.92, 1.67).2,22
-
†
In the EMPA-KIDNEY trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6609 patients with CKD, the efficacy and safety of JARDIANCE® 10 mg (n=3304) were evaluated vs placebo (n=3305). The primary endpoint in the EMPA-KIDNEY trial was a composite of CV death or progression of kidney disease. Patients treated with JARDIANCE® experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64, 0.82; p<0.001).3
-
‡
In the EMPEROR-Reduced trial, a randomised, double-blind, parallel-group, placebo-controlled study of 3730 patients with HFrEF, the efficacy and safety of JARDIANCE® 10 mg (n=1863) were evaluated vs placebo (n=1867). Patients were adults with chronic HF (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤40%). The primary endpoint in the EMPEROR-Reduced trial was a composite of CV death or hospitalisation for HF, analysed as time to the first event. Patients treated with JARDIANCE® experienced a 25% RRR in this endpoint (HR=0.75; 95% CI: 0.65, 0.86; p<0.001).4
-
§
In the EMPEROR-Preserved trial, a randomised, double-blind, parallel-group, placebo-controlled study of 5988 patients with HFpEF, the efficacy and safety of JARDIANCE® 10 mg (n=2997) were evaluated vs placebo (n=2991). Patients were adults with chronic HF (NYHA class II, III, or IV) and preserved ejection fraction (LVEF >40%). The primary endpoint in the EMPEROR-Preserved trial was a composite of CV death or hospitalisation for HF, analysed as time to the first event. Patients treated with JARDIANCE® experienced a 21% RRR in this endpoint (HR=0.79; 95% CI: 0.69, 0.90; p<0.001).5
-
||
In the EMPULSE trial, a randomised, double-blind, placebo-controlled study of 530 patients with chronic heart failure regardless of LVEF, the efficacy and safety of JARDIANCE® 10 mg (n=265) were evaluated vs placebo (n=265). The primary endpoint in the EMPULSE trial was clinical benefit, defined as a hierarchical composite of death from any cause, number of heart failure events and time to first heart failure event, or a 5-point or greater difference in change from baseline in the KCCQ-TSS at 90 days, as assessed using a win ratio. Patients treated with JARDIANCE® experienced an overall clinical benefit 36% more likely than with placebo (win ratio 1.36; 95% CI: 1.09, 1.68; p=0.0054).2
-
¶
Adult patients with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke.2
-
#
In addition to reducing the risk of CV death when added to the standard of care, JARDIANCE® also lowered HbA1c. In addition, JARDIANCE® demonstrated reduction in weight and blood pressure. JARDIANCE® is not indicated for weight loss or reduction of blood pressure.2
-
**
CV death was part of the composite primary endpoint, 3-point MACE, in the EMPA-REG OUTCOME® trial (HR=0.86; 95% CI: 0.74, 0.99; p<0.001 for noninferiority; p=0.04 for superiority) and 38% RRR in CV death was achieved in the overall EMPA-REG OUTCOME® population for the duration of the trial (HR=0.62; 95% CI: 0.49, 0.77; p<0.001). There were no significant differences between the placebo and JARDIANCE® groups of nonfatal MI (HR=0.87; 95% CI: 0.70, 1.09; p=0.22) or nonfatal stroke (HR=1.24; 95% CI: 0.92, 1.67; p=0.16).2
-
††
Pooled data from 10-mg and 25-mg doses of JARDIANCE®; both doses showed a comparable reduction in the risk of CV death.2
-
‡‡
Standard of care included CV medications and glucose-lowering agents given at the discretion of healthcare providers and according to recommendations of local guidelines.2
-
§§
A post hoc analysis of data from the EMPA-REG OUTCOME® trial by baseline HbA1c subgroups. EMPA-REG OUTCOME® was not powered to show differences between subgroups.2,19
-
||||
According to nonparametric age-based Kaplan-Meier estimates of the survival curve, which were based on actuarial estimates of the age-specific probabilities of death for the pooled JARDIANCE® group and the placebo group in the EMPA-REG OUTCOME® trial. At 45 years of age, the estimated mean survival was 32.1 years in the JARDIANCE® group vs 27.6 years in the placebo group (difference, 4.5 years; 95% CI: 1.3, 7.8; p=0.007).20
-
ACEi,
angiotensin-converting enzyme inhibitor; ARR, absolute risk reduction; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; DBP, diastolic blood pressure; eCVD, established cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; HHF, hospitalization for heart failure; KCCQ-TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; MI, myocardial infarction; NNT, number needed to treat; NYHA, New York Heart Association; PAD, peripheral artery disease; PCP, primary care physician; RRR, relative risk reduction; SBP, systolic blood pressure; SGLT2i, sodium-glucose cotransporter-2 inhibitor; T2D, type 2 diabetes.
PC-IN-103695