Patient profile

HOW WOULD YOU HELP PROTECT PATIENTS LIKE KARAN?
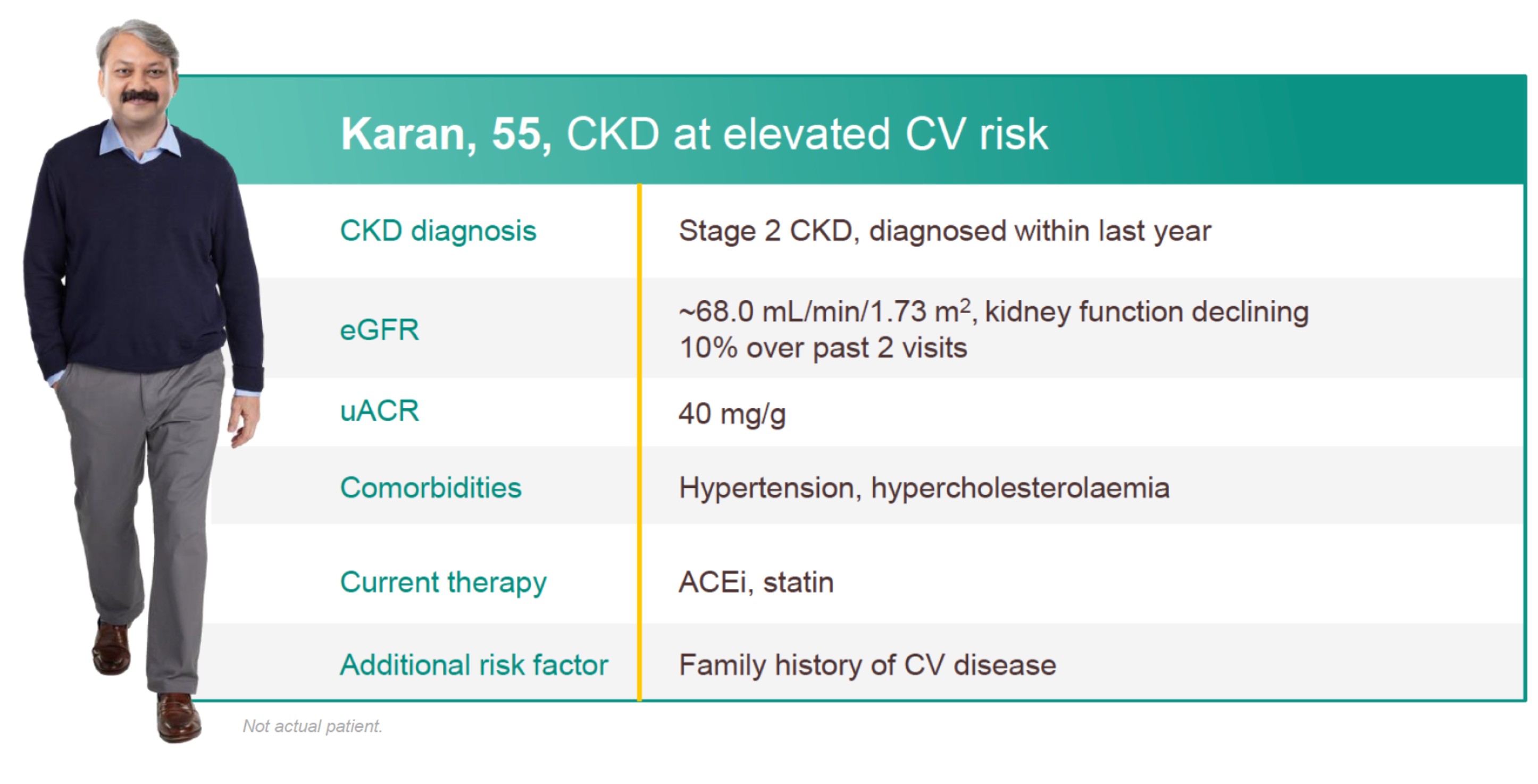
Find out how JARDIANCE can help protect patients like Karan by reducing kidney disease progression or risk of CV death*†1,2
-
*
Adult patients with an eGFR ≥30, <45 mL/min/1.73 m2; or an eGFR ≥45, <90 mL/min/1.73 m2 with a uACR ≥200 mg/g.2
-
†
In the EMPA-KIDNEY trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6609 patients with CKD, the efficacy and safety of JARDIANCE 10 mg (n=3304) were evaluated vs placebo (n=3305). The primary endpoint in the EMPA-KIDNEY trial was a composite of CV death or progression of kidney disease. Patients treated with JARDIANCE experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64, 0.82; p<0.001).2
-
ACEi=angiotensin-converting enzyme inhibitor; CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HR=hazard ratio; RRR=relative risk reduction; uACR=urinary albumin-to-creatinine ratio.
-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
-
Herrington WG, Staplin N, Wanner C, et al. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix.)
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D*
JARDIANCE REDUCED KIDNEY DISEASE PROGRESSION OR RISK OF CV DEATH IN A BROAD PATIENT POPULATION†1,2
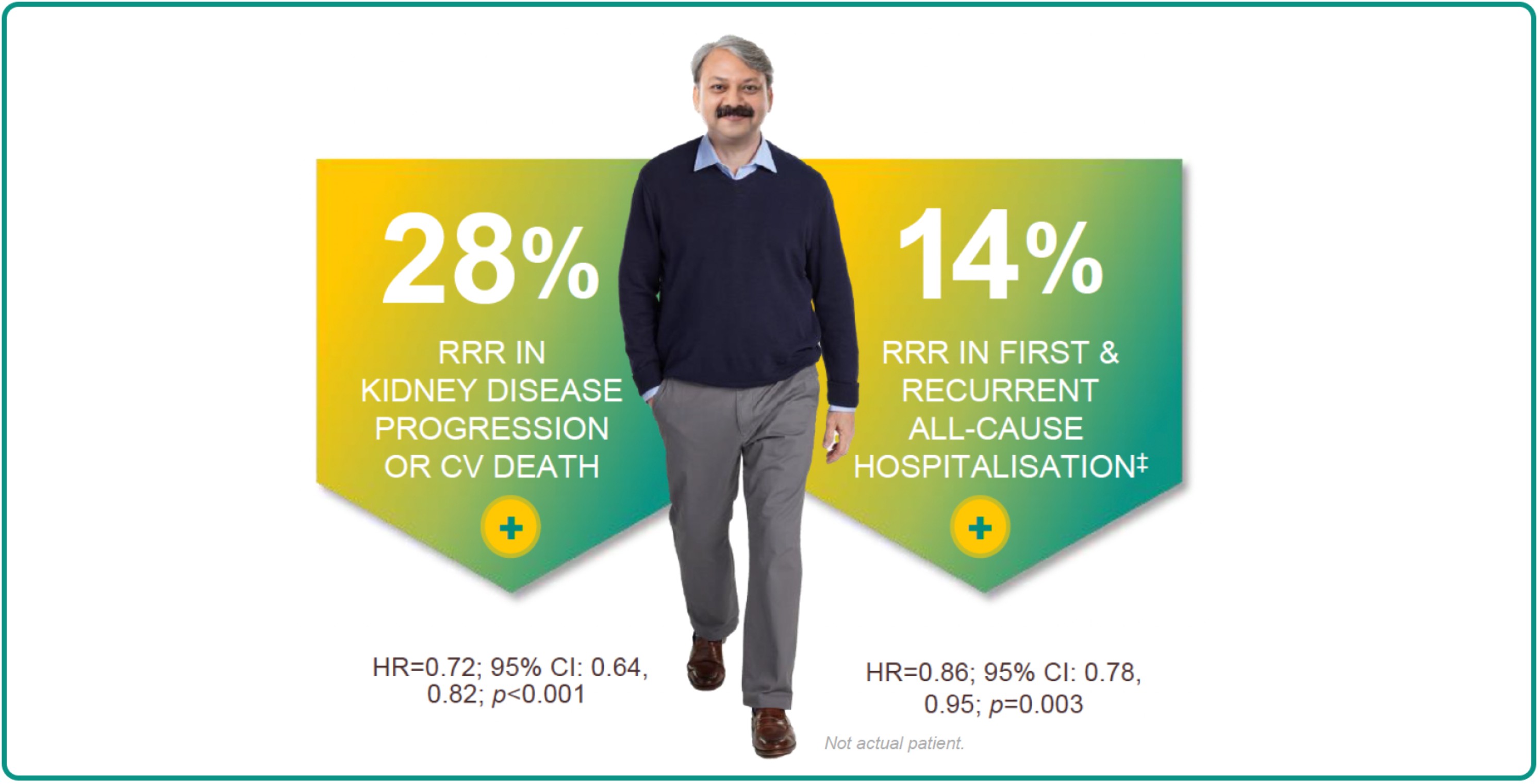
Consistent benefits proven across the patients you see in your practice including2:
With and without T2D
Mildly to severely reduced eGFR (<90 to 30 mL/min/1.73 m2)
Across a spectrum of uACR (<30 to >300 mg/g)
With or without background use of RAASi
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D*
JARDIANCE REDUCED KIDNEY DISEASE PROGRESSION OR RISK OF CV DEATH2
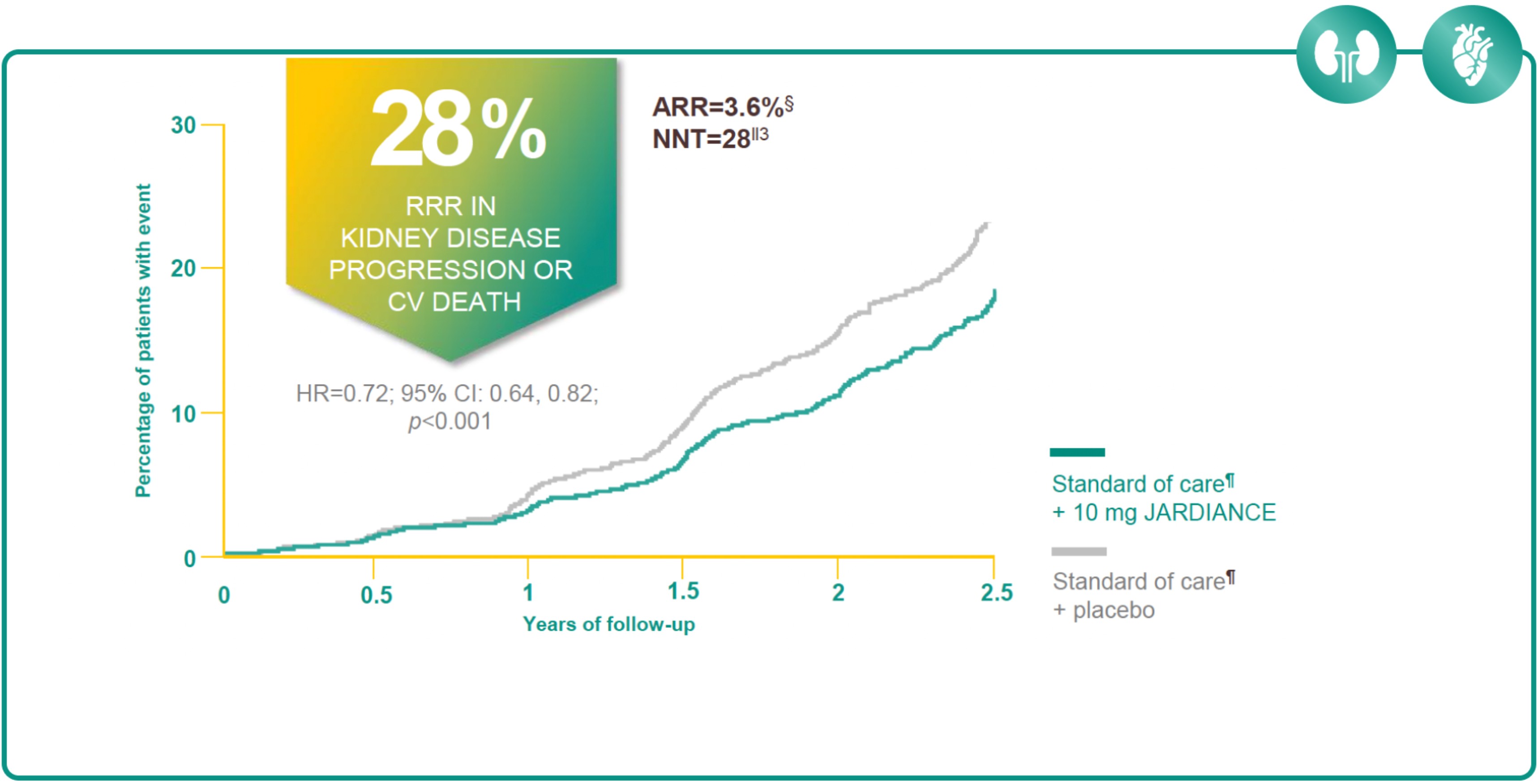
Results from the EMPA-KIDNEY trial.
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D*
JARDIANCE HELPED PROTECT PATIENTS BY GIVING MORE TIME OUT OF HOSPITAL2
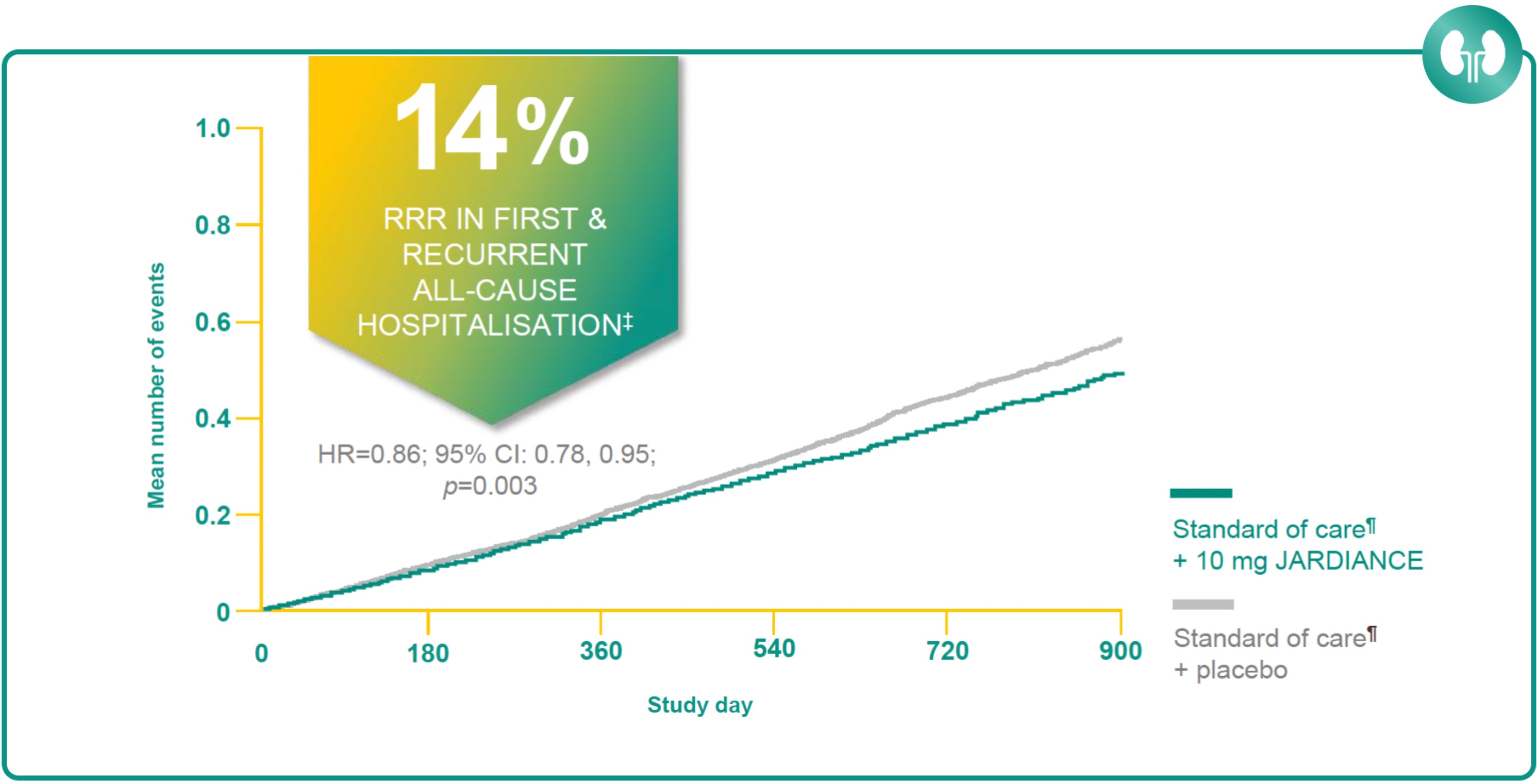
Results from the EMPA-KIDNEY trial.
-
*
Adult patients with an eGFR ≥30, <45 mL/min/1.73 m2; or an eGFR ≥45, <90 mL/min/1.73 m2 with a uACR ≥200 mg/g.2
-
†
In the EMPA-KIDNEY trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6609 patients with CKD, the efficacy and safety of JARDIANCE 10 mg (n=3304) were evaluated vs placebo (n=3305). The primary endpoint in the EMPA-KIDNEY trial was a composite of CV death or progression of kidney disease. Patients treated with JARDIANCE experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64, 0.82; p<0.001).2
-
‡
Hospitalisation for any cause was a key secondary outcome of the EMPA-KIDNEY trial. The analysis of hospitalisations for any cause included the first and all subsequent events (JARDIANCE, 1611 hospitalisations in 960 patients; placebo, 1895 hospitalisations in 1035 patients).2
-
§
ARR for the primary composite outcome of kidney disease progression or CV death is 3.6% per patient-year at risk. Figure adapted from Herrington et al.2
-
ll
NNT: 28 (95% CI: 19, 53) per 2 years at risk.3
-
¶
Standard of care: All patients received a RAASi unless an investigator judged that a RAASi was not indicated or tolerated.2
-
ARR=absolute risk reduction; CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HR=hazard ratio; NNT=number needed to treat; RAASi=renin-angiotensin-aldosterone system inhibitor; RRR=relative risk reduction; T2D=type 2 diabetes; uACR=urinary albumin-to-creatinine ratio.
-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
-
Herrington WG, Staplin N, Wanner C, et al. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix.)
-
Data on file. Boehringer Ingelheim Pharmaceuticals, Inc. 2022.
