Patient profile

IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D*
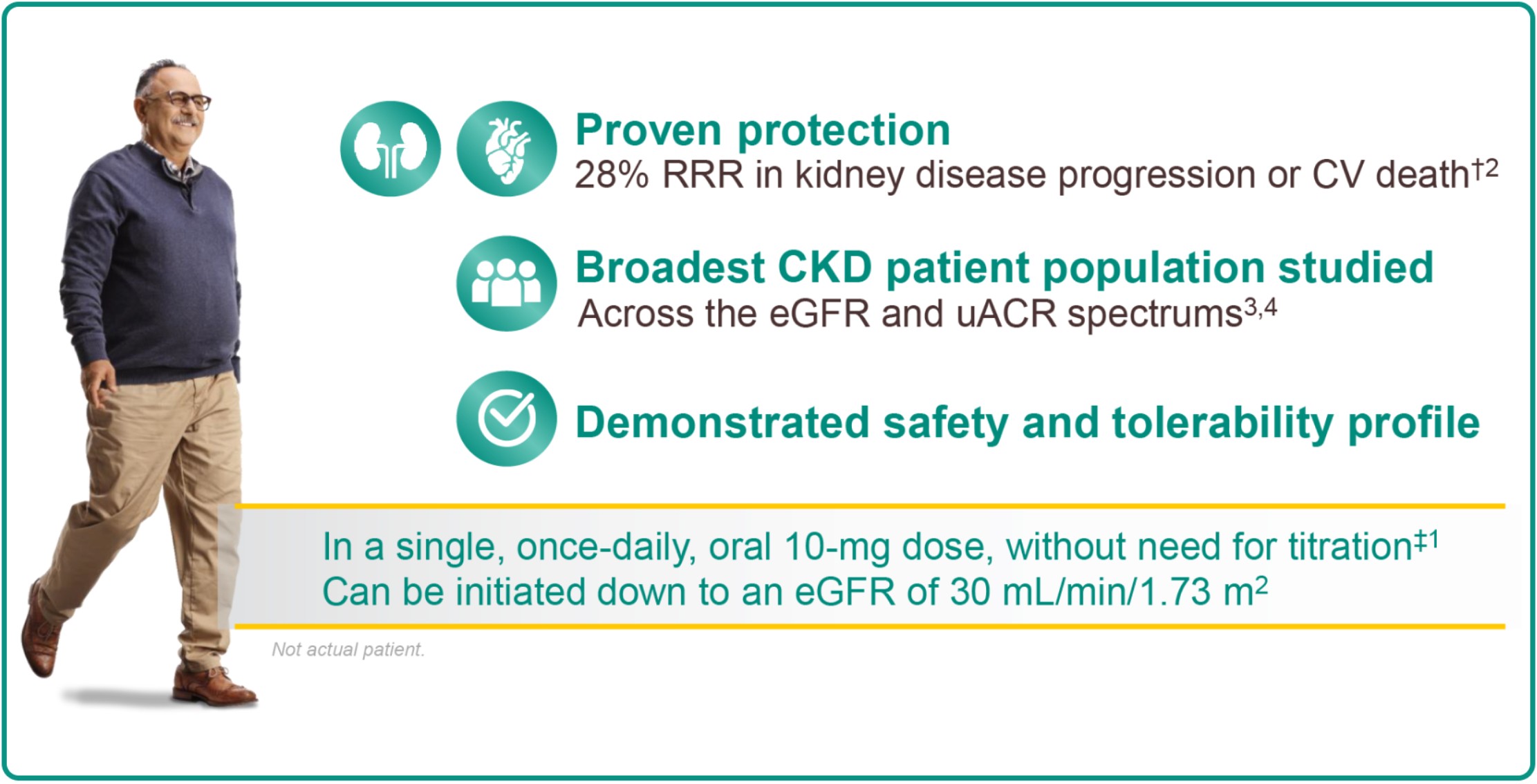
HELP PROTECT KETAN’S KIDNEYS AND HEART
Prescribe JARDIANCE today to reduce his kidney disease progression or risk of CV death1
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D*
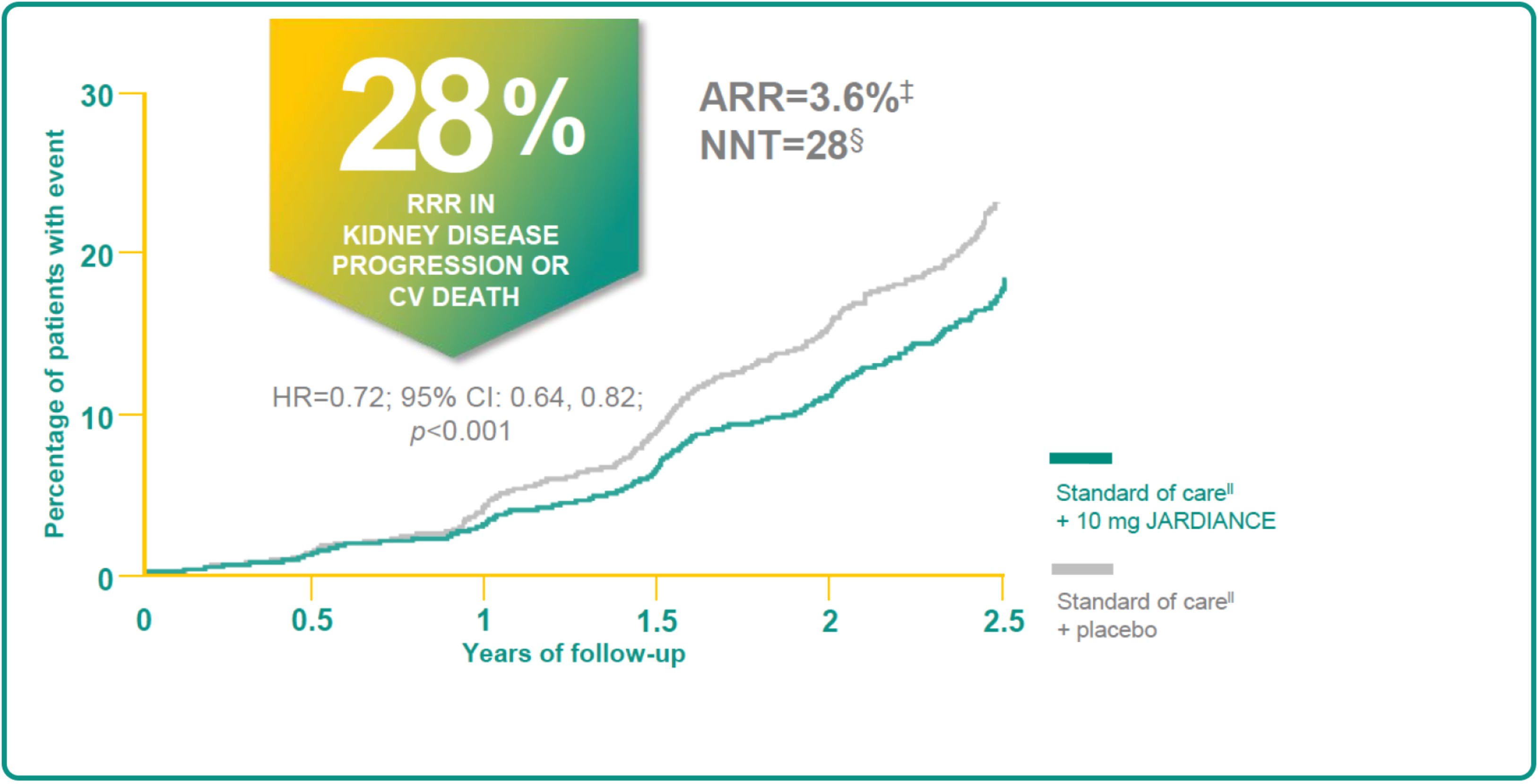
JARDIANCE REDUCED KIDNEY DISEASE PROGRESSION OR RISK OF CV DEATH†1,2
Protect the kidneys and heart across your broad range of patients with CKD2:
With and without T2D
With or without background use of RASi
FOR PATIENTS WITH TYPE 2 DIABETES AND CV DISEASE
IN EMPA-REG OUTCOME STUDY,¶ JARDIANCE RESULTED IN A SIGNIFICANTLY LOWER RISK OF ALL-CAUSE MORTALITY COMPARED WITH PLACEBO1,3
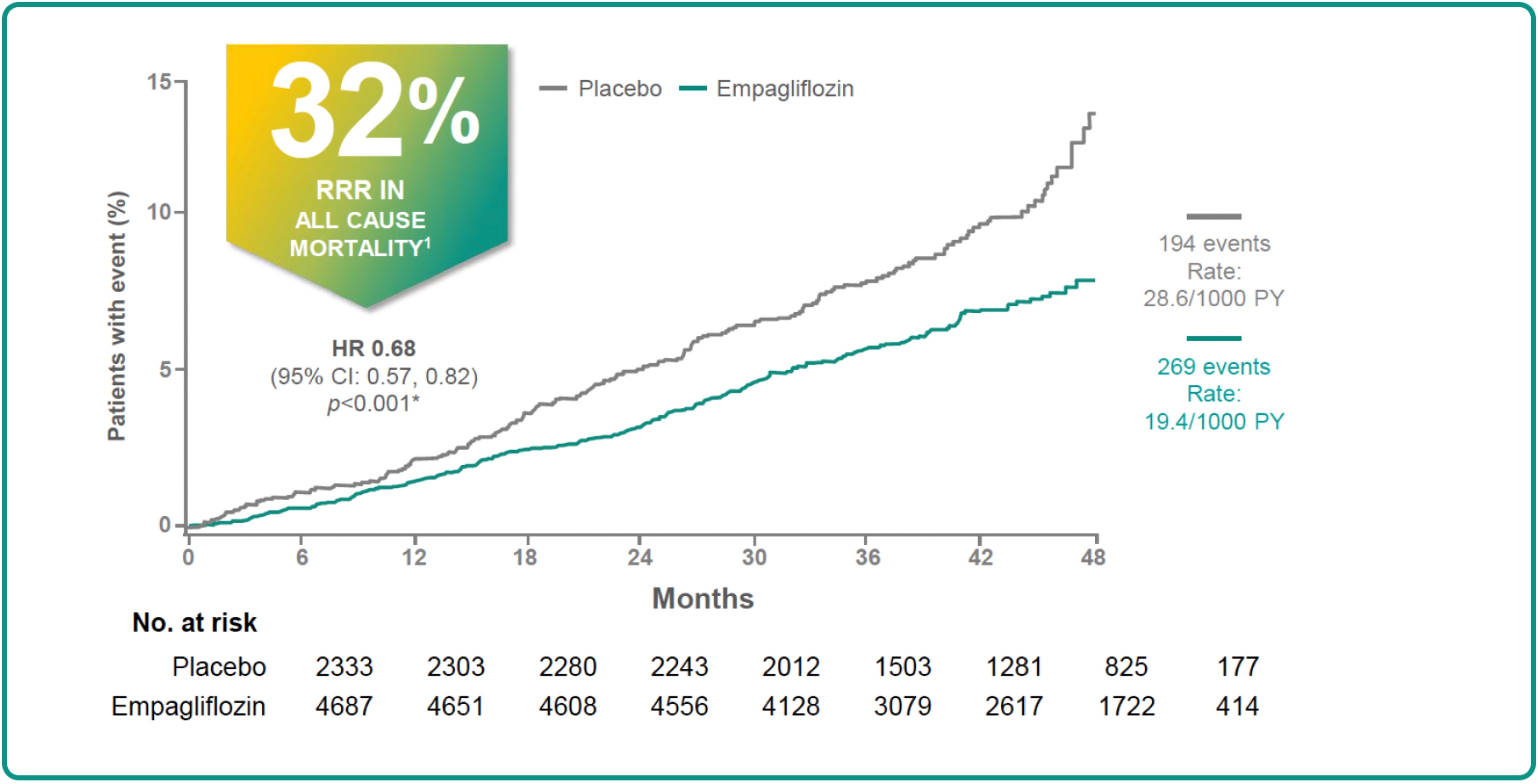
Results from the EMPA-REG OUTCOME® trial.
-
*
Adult patients with eGFR ≥45 to <90 mL/min/1.73 m2 with UACR ≥200 mg/g, or eGFR ≥30 to <45 mL/min/1.73 m2.
-
†
In the EMPA-KIDNEY trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6609 patients with CKD, the efficacy and safety of JARDIANCE 10 mg (n=3304) were evaluated vs placebo (n=3305). The primary endpoint in the EMPA-KIDNEY trial was a composite of CV death or progression of kidney disease (defined as end-stage kidney disease; the initiation of maintenance dialysis or receipt of a kidney transplant). Patients treated with JARDIANCE experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64, 0.82; p<0.001).2
-
‡
ARR for the primary composite outcome of the kidney disease progression or CV death is 3.6% per patient-year at risk. Figure adapted from Herrington et al.2
-
§
NNT : 28 (95% Cl: 19, 53) per 2 year at risk.2
-
ll
Standard of care: All patients received a RAASi unless an investigator judged that a RAASi was not indicated or tolerated.2
-
¶
EMPA-REG OUTCOME study design: A randomized, double-blind, parallel-group trial comparing the risk of experiencing a major adverse cardiovascular event between Jardiance® (empagliflozin) tablets and placebo when these were added to and used concomitantly with standard-of-care treatments for type 2 diabetes and cardiovascular disease. A total of 7020 patients were treated (JARDIANCE 10 mg [n=2345]; JARDIANCE 25 mg [n=2342]; placebo [n=2333]) and followed for a median of 3.1 years. All patients had established atherosclerotic cardiovascular disease at baseline, including one or more of the following: a documented history of coronary artery disease, peripheral artery disease, myocardial infarction, or stroke. The primary outcome was a reduction in the risk of cardiovascular events, defined by the composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke.3
-
ARR=absolute risk reduction; CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HR=hazard ratio; NNT=number needed to treat; RAASi=renin-angiotensin-aldosterone system inhibitor; RRR=relative risk reduction; T2D=type 2 diabetes; uACR=urinary albumin-to-creatinine ratio.
-
"Jardiance prescribing information; version 18th Jul 2024; Boehringer Ingelheim India.“
-
Herrington WG, Staplin N, Wanner C, et al. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix.)
-
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D*
JARDIANCE DEMONSTRATES CONSISTENT KIDNEY AND CV EFFICACY OUTCOMES ACROSS THE BROADEST SPECTRUM OF SUBGROUPS†1
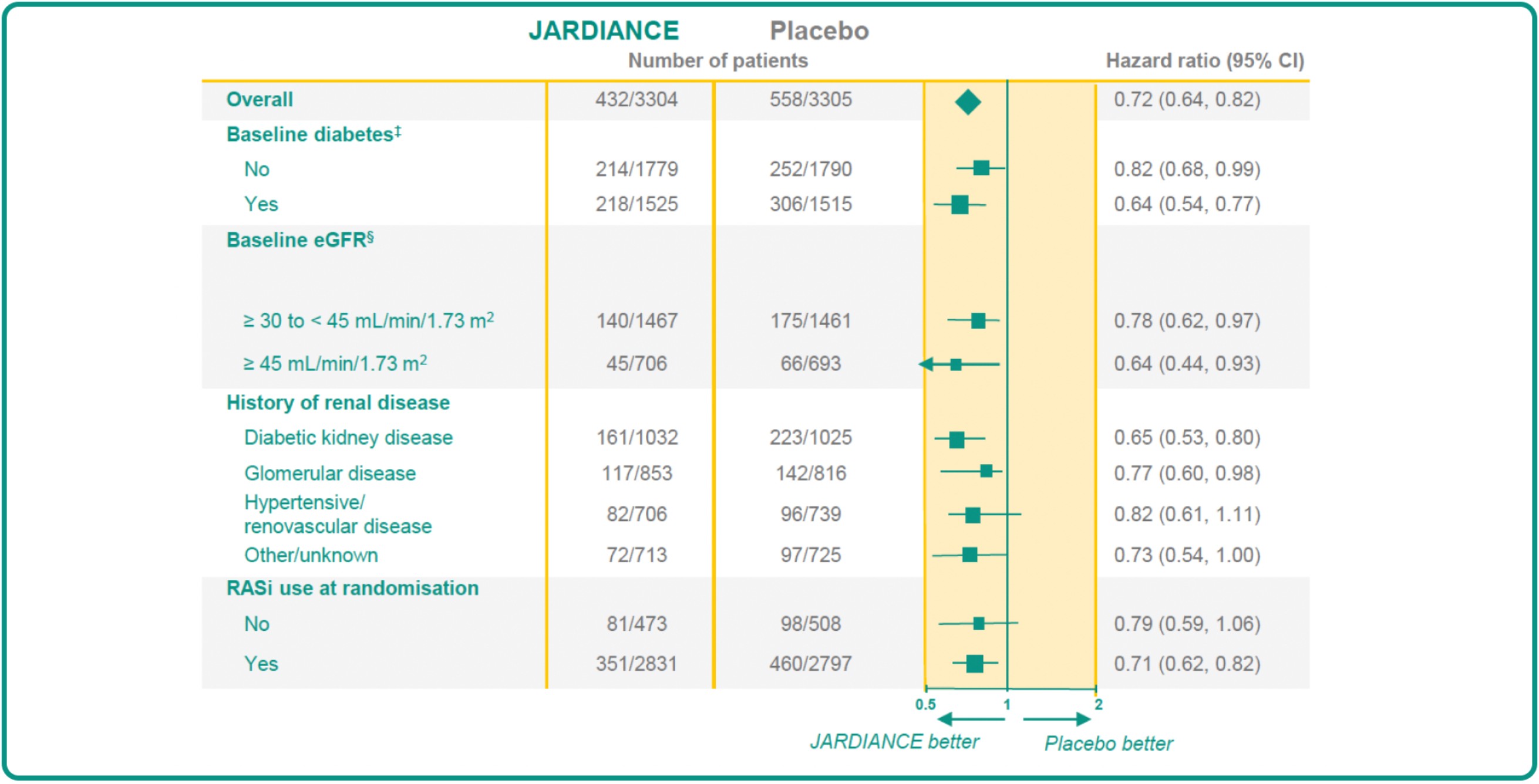

JARDIANCE is appropriate for a broad CKD patient population* you see every day in your practice
-
*
Adult patients with an eGFR ≥45 to <90 mL/min/1.73 m2 with UACR ≥200 mg/g, or eGFR ≥30 to <45 mL/min/1.73 m2.1
-
†
In the EMPA-KIDNEY trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6609 patients with CKD, the efficacy and safety of JARDIANCE 10 mg (n=3304) were evaluated vs placebo (n=3305). The primary endpoint in the EMPA-KIDNEY trial was a composite of CV death or progression of kidney disease (defined as end-stage kidney disease; the initiation of maintenance dialysis or receipt of a kidney transplant). Patients treated with JARDIANCE experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64, 0.82; p<0.001).1
-
‡
History of diabetes was defined as a patient-reported history of diabetes of any type, use of glucose-lowering medication, or a glycated haemoglobin level of at least 48 mmol per mole (6.5%) at the randomisation visit. More than 95% of patients with diabetes had type 2.1
-
§
Values represent the measurement recorded at the randomisation visit or the most recent local laboratory result recorded before randomisation.1
-
CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HR=hazard ratio; RASi=renin-angiotensin system inhibitor; RRR=relative risk reduction; T2D=type 2 diabetes; uACR=urine albumin-to-creatinine ratio.
-
Herrington WG, Staplin N, Wanner C, et al. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix.)
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D*
JARDIANCE PROTECTS THE KIDNEYS BY SLOWING THE DECLINE IN RENAL FUNCTION OVER TIME†‡1
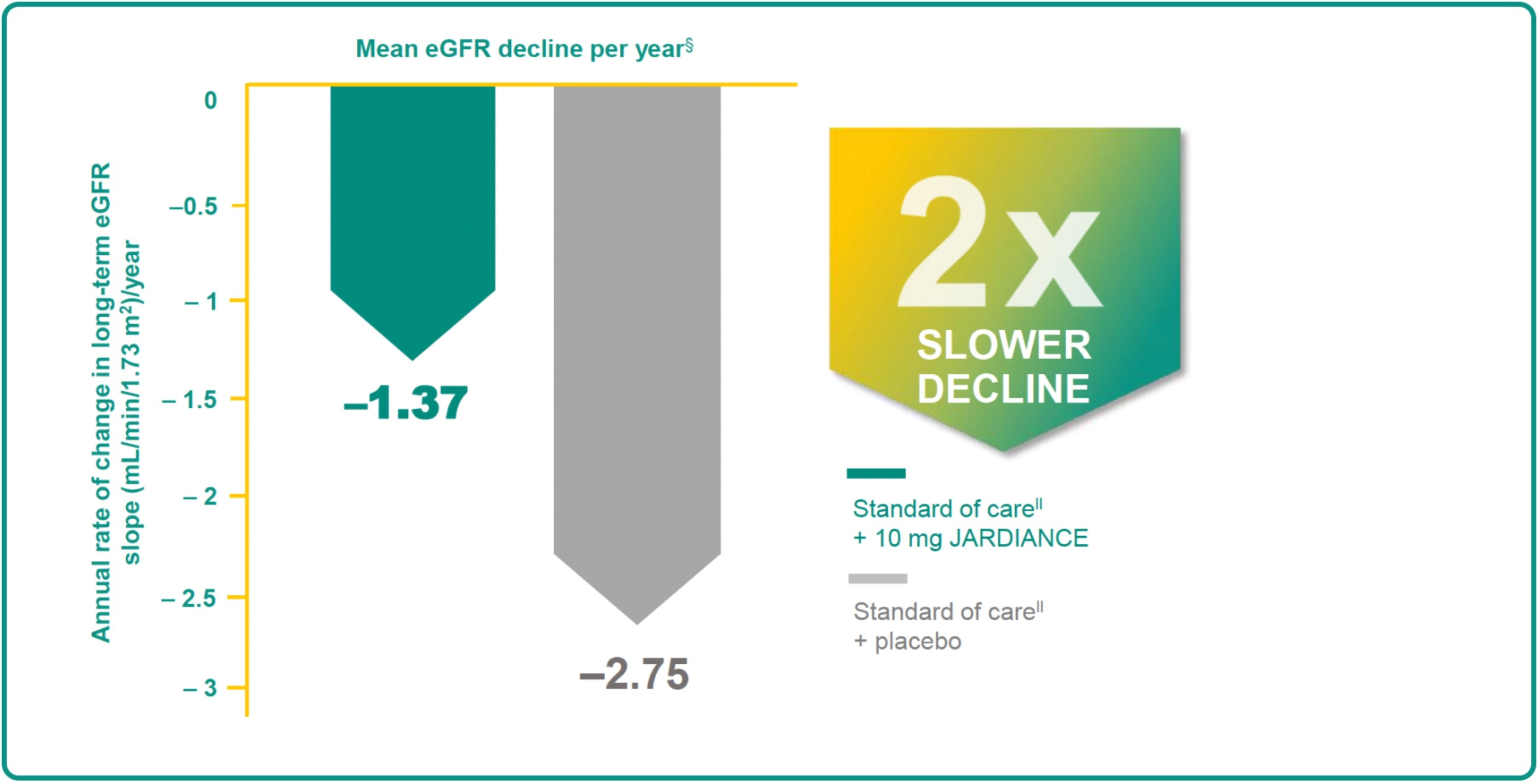
prolong better kidney function vs standard of
care#
IN THE TREATMENT OF PATIENTS WITH CKD WITH OR WITHOUT T2D*
PRESCRIBE JARDIANCE AS FOUNDATIONAL AND BROAD TREATMENT TO HELP PROTECT THE KIDNEYS AND THE HEART1,2