Dosing and administration
JARDIANCE can be initiated down to an eGFR of 301
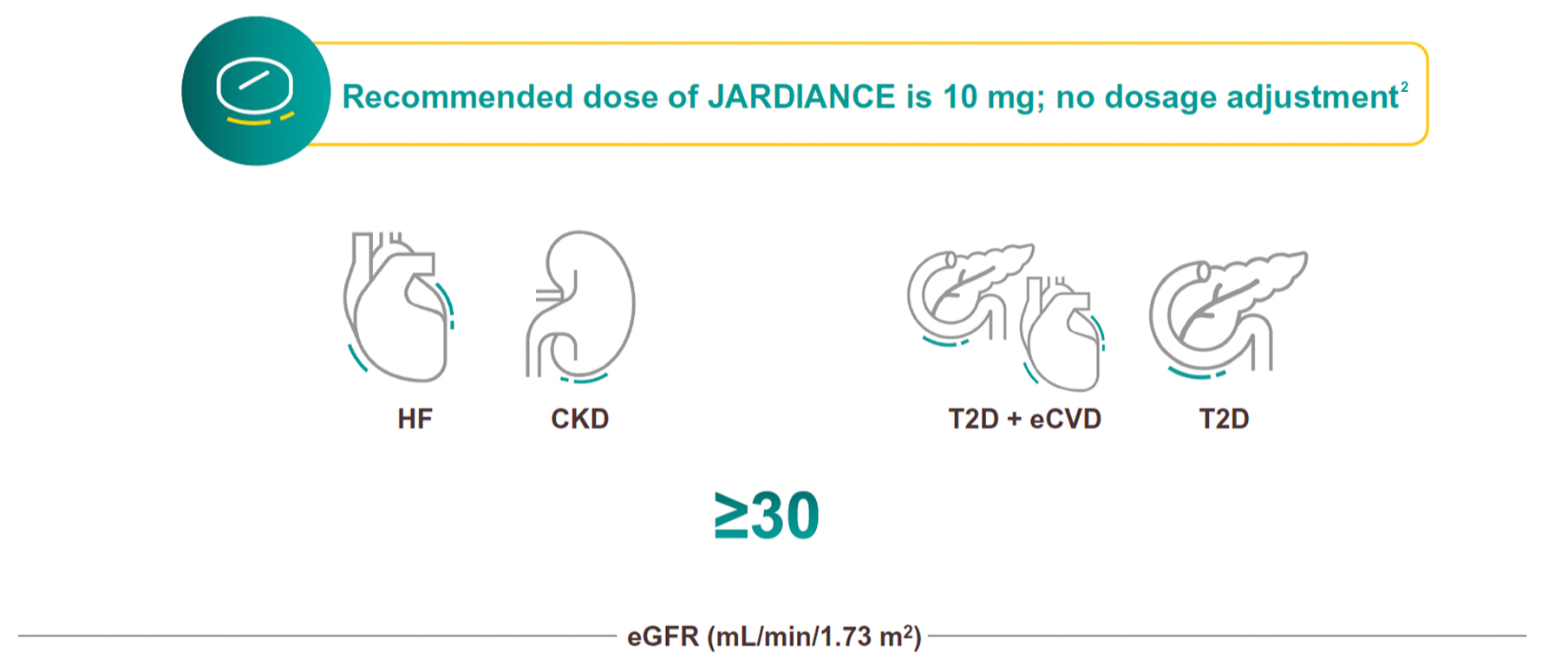
For full initiation and dosing details for patients with T2D with and without CVD, please refer to the Indian Prescribing Information.
-
*
When JARDIANCE is used in combination with a sulphonylurea or with insulin, a lower dose of the sulphonylurea or insulin may be considered to reduce the risk of hypoglycaemia.1
-
†
Please see the Indian Prescribing Information for the full list of AEs and full list of special warnings and precautions for use.
-
‡
Mean percentage increase.
-
AE=adverse event; CKD=chronic kidney disease; CV=cardiovascular; CVD=cardiovascular disease; eCVD=established cardiovascular disease; eGFR=estimated glomerular filtration rate; HF=heart failure; SmPC=Summary of Product Characteristics; T2D=type 2 diabetes.
-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
-
Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568-574.
JARDIANCE is the obvious choice as first-line heart failure treatment if starting on all 4 pillars is not an option
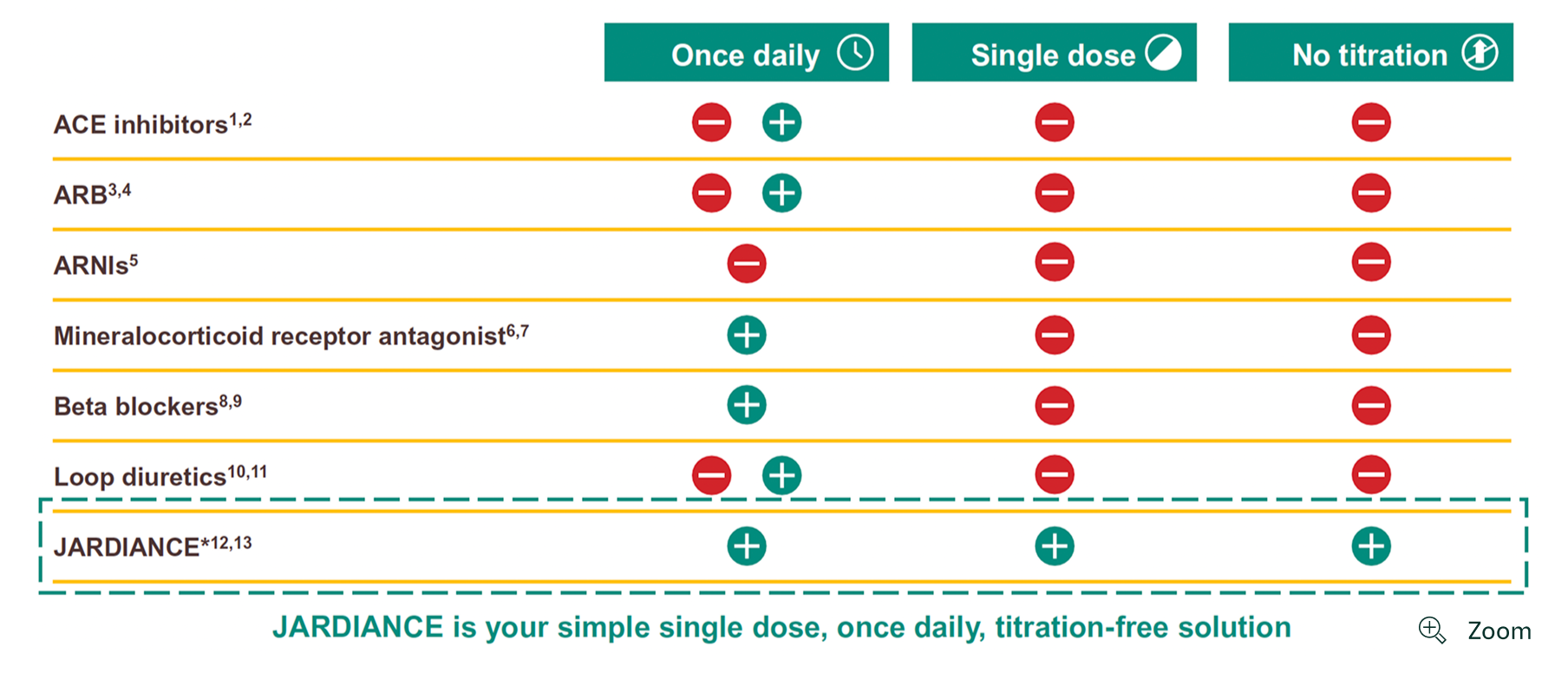
-
*
For patients with type 2 diabetes treated with empagliflozin who need tighter glycemic control, the dose may be increased.13
-
ACEi=angiotensin-converting enzyme inhibitor; ARB=angiotensin II receptor blocker; ARNI=angiotensin receptor neprilysin inhibitor; SGLT2=sodium-glucose co-transporter-2.
-
Valeant Pharmaceuticals International, Inc. VASOTECTM Prescribing Information, 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/018998s0831bl.pdf.
-
Pfizer lnc. ALTACE® Prescribing Information, 2017. Available at: http://labelling.pfizer.com/showlabeling.aspx?id=716.
-
Novartis Pharmaceuticals Corporation. DIOVAN® Prescribing Information, 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021283s058lbl.pdf.
-
ANI Pharmaceuticals, Inc. ATACAND® Prescribing Information, 2020. Available at: https://wwww.accessdata.fda.gov/drugsatfda_docs/label/2020/020838s041lbl.pdf.
-
Novartis Pharmaceuticals Corporation. ENTRESTO® Prescribing Information, 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/207620s018lbl.pdf.
-
Pfizer Inc. ALDACTONE® Prescribing Information, 2021. Available at: http://labelling.Pfizer.com/ShowLabeling.aspx?format=PDF&id=520.
-
Pfizer Inc. INSPRA® Prescribing Information, 2020. Available at: http://labelling.Pfizer.com/showlabeling.aspx?id=599.
-
Accord Healthcare Limited. Bisoprolol SmPC, 2022. Available at: http://www.medicines.org.uk/emc/product/8850/smpc.
-
GlaxoSmithKline. COREG CR Prescribing Information, 2020. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022012s010s013lbl.pdf.
-
Mylan. TOREM® SmPC, 2018. Available at: https://www.medicines.org.uk/emc/product/6663/smpc.
-
Accord Healthcare Ltd. Furosemide SmPC, 2016. Available at: https://www.medicines.org.uk/emc/product/6012/smpc/print.
-
AstraZeneca Pharmaceuticals LP. FORXlGA® SmPC, 2022. Available at: https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf.
-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
