Patient profile

Rajeev, 68
Referred to you by his PCP for cardiac review


HFrEF

- Hypercholesterolaemia
- NYHA class II

- Statin
58

30%-35%

115/65

Add JARDIANCE early to reduce his risk of CV death or HHF
-
*
Adult patients with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤40%).1-3
-
†
Adult patients with chronic heart failure (NYHA class II, III, or IV) and preserved ejection fraction (LVEF >40%).2
-
CV=cardiovascular; eGFR=estimated glomerular filtration rate; HF=heart failure; HFpEF=heart failure with preserved ejection fraction; HFrEF=heart failure with reduced ejection fraction; HHF=hospitalisation for heart failure; LVEF=left ventricular ejection fraction; NYHA=New York Heart Association; PCP=primary care physician.
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. (EMPEROR-Preserved results and the publication’s Supplementary Appendix.)
-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
In the treatment of patients with HF with LVEF ≤40%*

In the treatment of patients with HF with LVEF ≤40%*
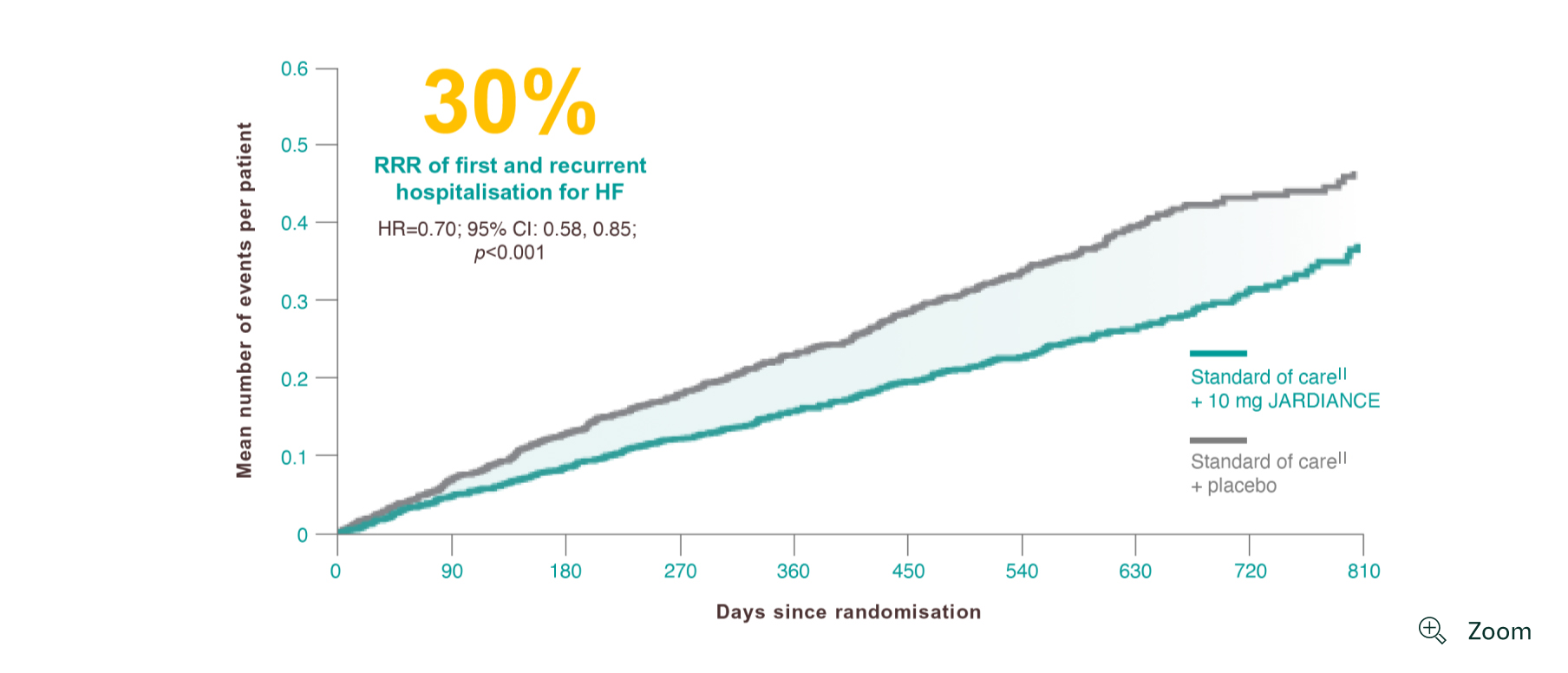
Results from the EMPEROR-Reduced trial.
-
*
Adult patients with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤40%).1
-
†
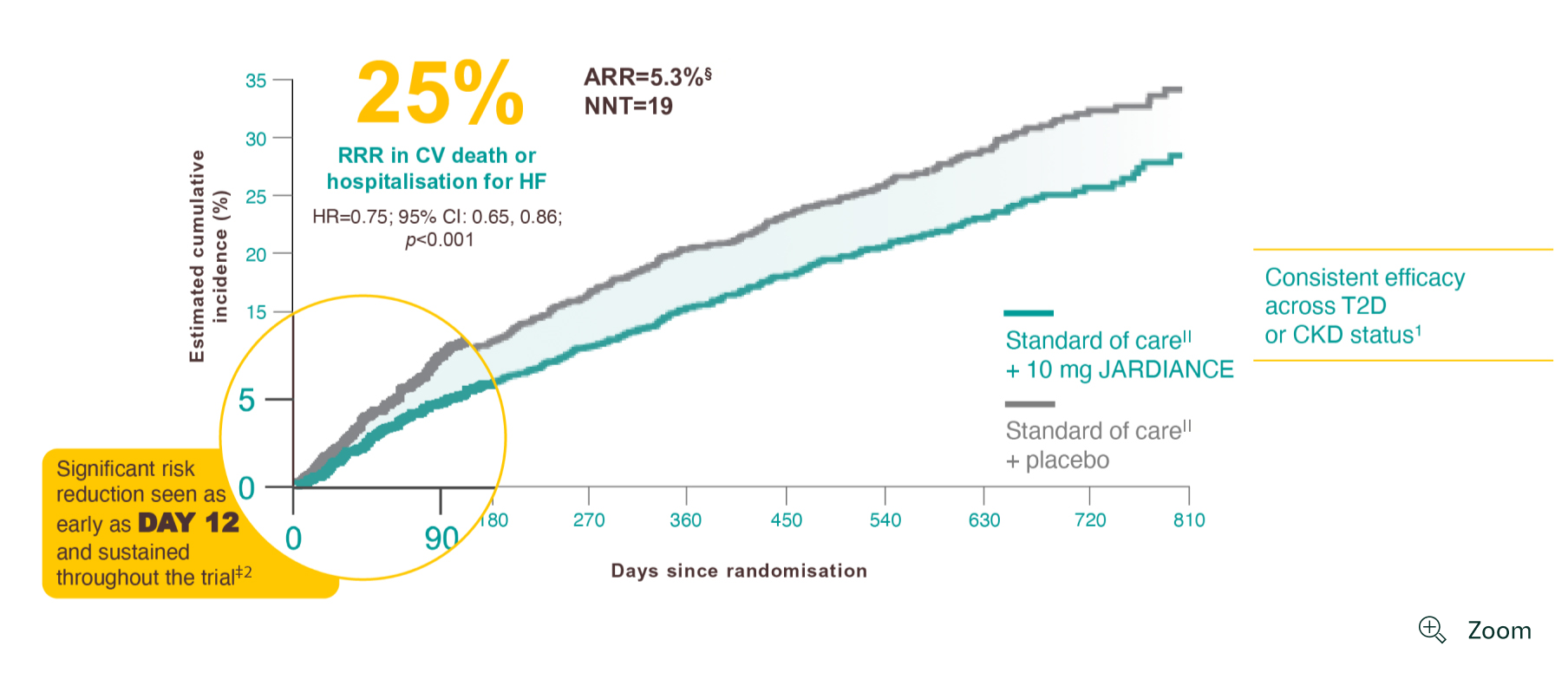
In the EMPEROR-Reduced trial, a randomised, double-blind, parallel-group, placebo-controlled study of 3730 patients with HFrEF, the efficacy and safety of JARDIANCE 10 mg (n=1863) were evaluated vs placebo (n=1867). The primary endpoint in the EMPEROR-Reduced trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE experienced a 25% RRR in this endpoint (HR=0.75; 95% CI: 0.65, 0.86; p<0.001).1
-
‡
Results from the EMPEROR-Reduced trial. The effect of empagliflozin to reduce the combined risk of death, hospitalisation for heart failure, or an emergent or urgent heart failure visit was statistically significant at 12 days after randomisation and statistical significance was sustained from day 12 onward (HR, 0.70; 95% CI: 0.63-0.78; p<0.0001).2
-
§
ARR calculation: JARDIANCE number of patients with events 361/total number of patients 1863=19.4%; placebo number of patients with events 462/total number of patients 1867=24.7%; 24.7%–19.4%=5.3%. NNT=1/ARR.1
-
II
Standard of care: All patients received appropriate treatments for heart failure, including diuretics, inhibitors of the renin-angiotensin system and neprilysin, beta blockers, mineralocorticoid receptor antagonists and, when indicated, cardiac devices.1
-
¶
The occurrence of all HHF, including first and recurrent events, was a prespecified secondary outcome of the EMPEROR-Reduced trial.1
-
#
In addition to the RRR of all HHF, including first and recurrent events, the risk of total hospitalisations for any reason was also significantly reduced (HR=0.85, 95% CI: 0.75, 0.95).1
-
ARR=absolute risk reduction; CV=cardiovascular; CKD=chronic kidney disease; HF=heart failure; HFpEF=heart failure with preserved ejection fraction; HFrEF=heart failure with reduced ejection fraction; HHF=hospitalisation for heart failure; HR=hazard ratio; LVEF=left ventricular ejection fraction; NNT=number needed to treat; NYHA=New York Heart Association.
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Committee and Investigators. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation. 2021;143(4):326-336.
In patients with stable HFrEF,
JARDIANCE® foundational therapy1
Rapid sequencing algorithm

How should we sequence the treatments for heart failure and reduced ejection fraction? A redefinition of evidence-based medicine.

Step 1: Simultaneous initiation of ß-blockers + SGLT2i
ß blockers are arguably most effective for risk of sudden death.
SGLT2i have a strikingly early benefit for HHF, may mitigate short-term risk of worsening HF with ß-blocker.

Step 2: Addition of ARNi, within 1-2 weeks of Step 1
if SBP <100 mmHG, evaluate tolerance in relation to hypotension with ARB, before switching to ARNi.
Risk involves with repeat dosing, or adjusting diuretic dose.

Step 3: Addition of MRA, within 1-2 weeks of step 2, if serum K+ is normal then renal function will not be severely impaired
Favourable effects of SGLT2i and ARNi to improve renal function and K+ homeostasis, may increase tolerability to MRA
MRAs may be step 2 in a patient with troublesome hypotension
-
SGLT2i=sodium glucose cotransporter 2 inhibitor; HF=heart failure; HHF=hospitalisation for heart failure; ARB=angiotensin receptor blockers; ARNi=angiotensin receptor neprilysin inhibitor; SBP=systolic blood pressure; MRA=mineralocorticoid receptor antagonist; HFrEF=heart failure with reduced ejection fraction.
-
Packer M, et al. Eur J Heart Fail. 2021;23(6):882-894.29.
-
McMurray JJV, et al. Circulation. 2021;143(9):875-7.
In non T2D patients with HF,
JARDIANCE® improves HF outcomes1,2 (EMPEROR Reduced and Preserved Studies)
CV Death or HHF outcomes in non-T2D HF patients
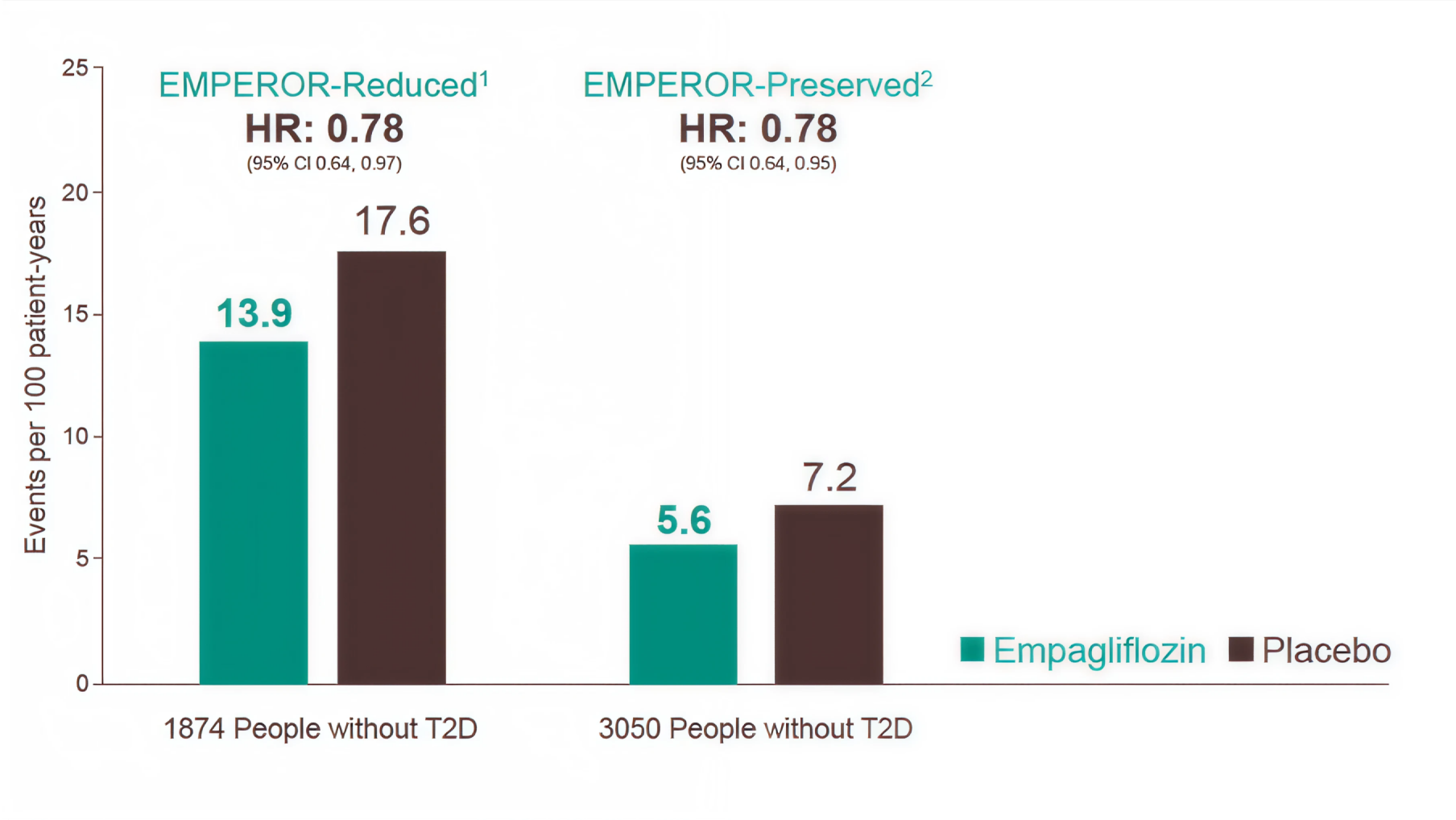
-
*
Percentages calculated using total number of patients per treatment as the denominator.
-
HF=heart failure; T2D=type 2 diabetes; CV=cardiovascular; HHF=hospitalisation for heart failure; HR=hazard ratio; CI=confidence interval.
-
Packer M, et al. N Engl J Med. 2020;383(15):1413-24.
-
Anker SD, et al. N Engl J Med. 2021;385(16):1451-1461.
In the treatment of HF patients with LVEF ≤40%II
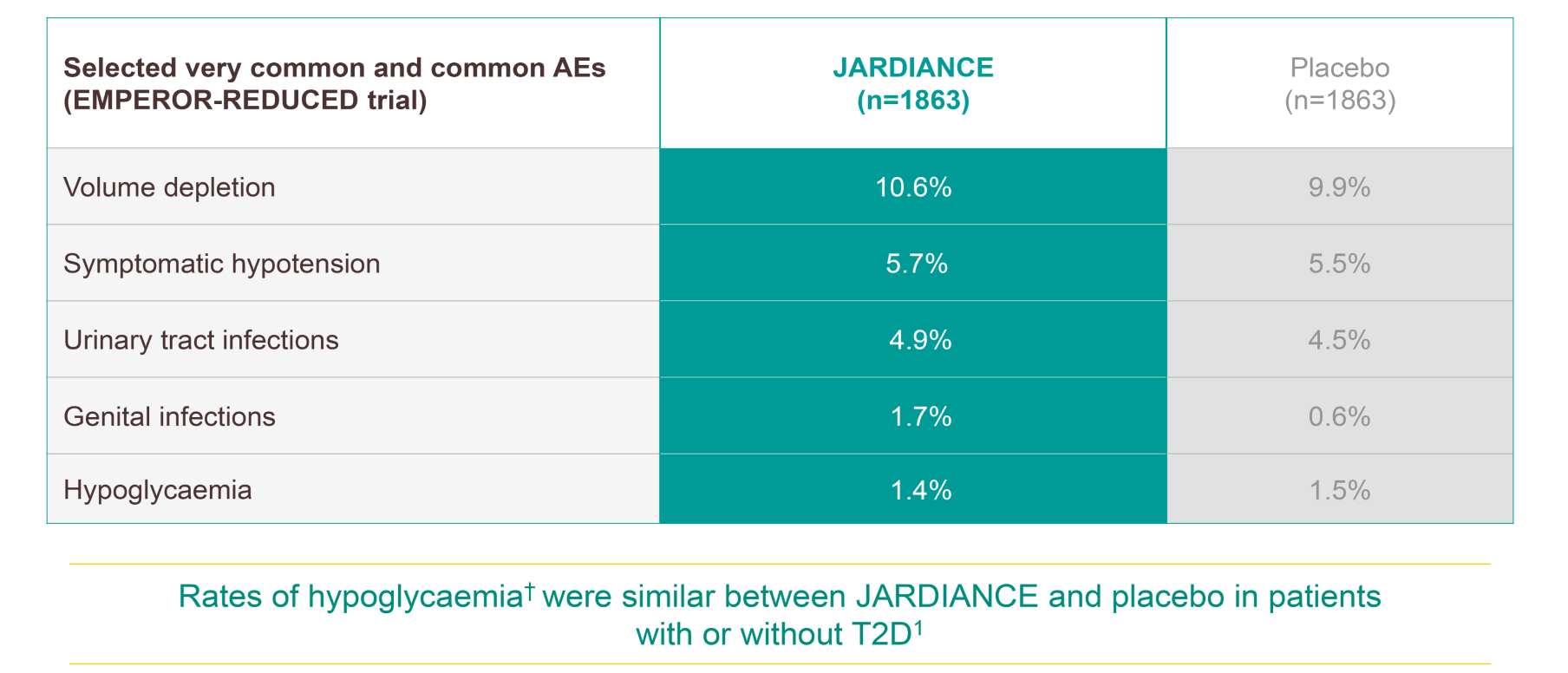
JARDIANCE has a proven safety and tolerability profile1
Rates of hypoglycaemia§ were similar between JARDIANCE and placebo in patients with or without T2D.1
-
*
Adult patients with chronic HF (NYHA class II, III, or IV) and preserved ejection fraction (LVEF ≤40%).1
-
†
Hypoglycaemic AEs with a plasma glucose value of ≤70 mg/dL (3.9 mmol/L) or that required assistance.1
-
AE=adverse event; HF=heart failure; LVEF=left ventricular ejection fraction; NYHA=New York Heart Association; T2D=type 2 diabetes.
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
PC-IN-103696 Validity till Oct 2025
