EMPEROR-Reduced trial results published in The New England Journal of Medicine in 20201
A randomised, double blind, placebo controlled, event driven trial

patients with LVEF ≤ 40%

countries

(median observation)

events observed
Prespecified efficacy endpoints
-
Composite primary endpoint: Time to first event of adjudicated CV death or adjudicated HHF
-
Confirmatory secondary endpoints: Total number of HHF, including first and recurrent; rate of decline in eGFR from baseline
Key inclusion criteria
-
Chronic heart failure (NYHA class II-IV)
-
Elevated NT-proBNP*
-
On appropriate doses of heart failure medication
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190 (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
*
Defined as a level of ≥ 1000 pg/mL in those with an ejection fraction of 31% to 35% or a level of ≥ 2500 pg/mL in those with an ejection fraction of 36% to 40%, as compared with a level of ≥ 600 pg/mL in those with an ejection fraction of ≤ 30%.1
-
†
Standard of care: All patients received appropriate treatments for heart failure, including diuretics, inhibitors of the renin-angiotensin system and neprilysin, beta blockers, mineralocorticoid receptor antagonists and, when indicated, cardiac devices.1
-
‡
Numbers shown are for the JARDIANCE arm.1
-
ACEi=angiotensin converting enzyme inhibitor; ARB=angiotensin II receptor blocker; ARNI=angiotensin receptor neprilysin inhibitor; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HHF= hospitalisation for heart failure; LVEF=left ventricular ejection fraction; MRA=mineralocorticoid receptor antagonist; NT proBNP=N terminal pro-brain natriuretic peptide; NYHA=New York Heart Association; T2D=type 2 diabetes.
EMPEROR-Reduced trial results published in The New England Journal of Medicine in 20201
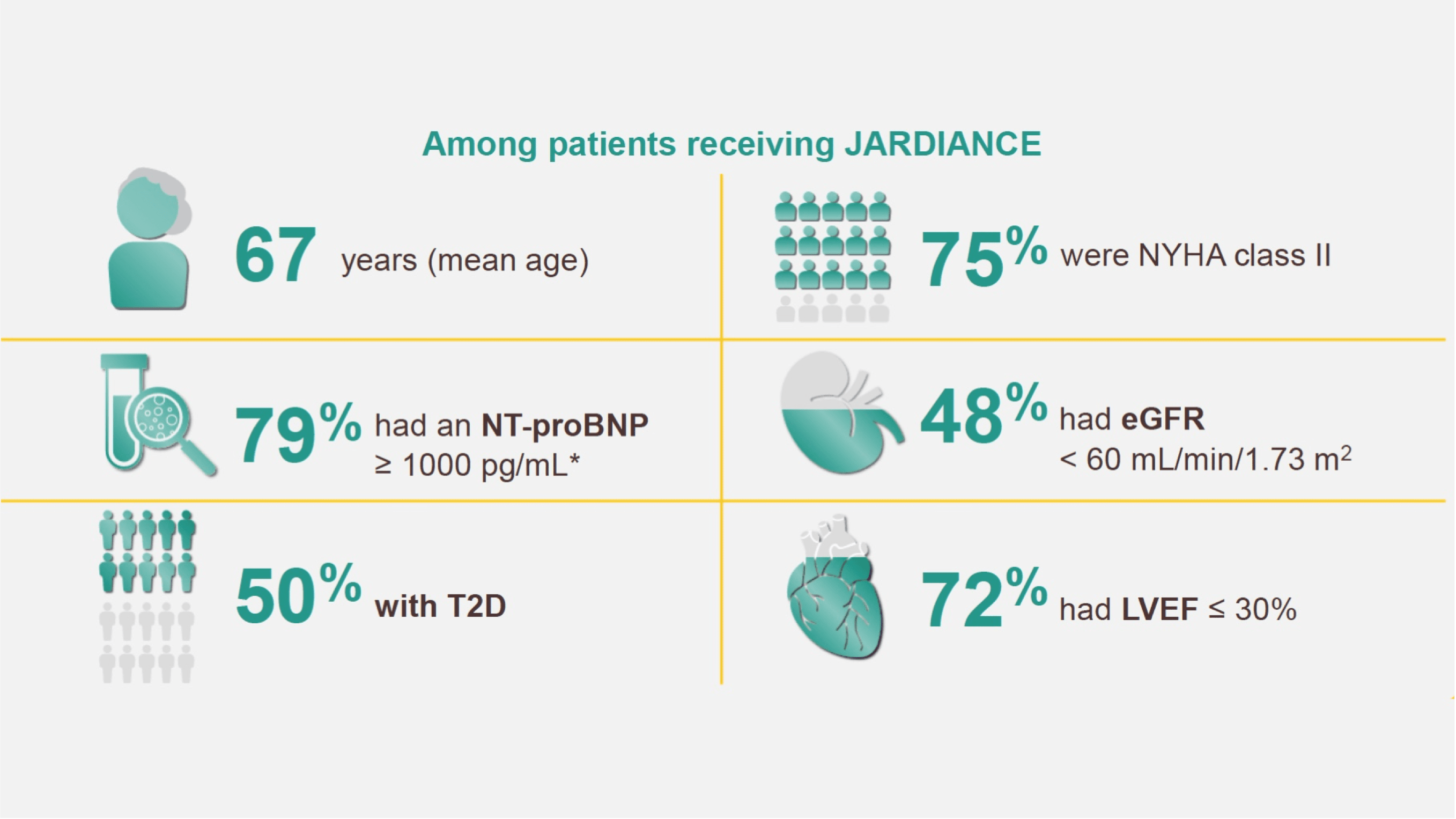
Patient characteristics

-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190 (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
*
Defined as a level of ≥ 1000 pg/mL in those with an ejection fraction of 31% to 35% or a level of ≥ 2500 pg /mL in those with an ejection fraction of 36% to 40%, as compared with a level of ≥ 600 pg /mL in those with an ejection fraction of ≤ 30%.1
-
†
Standard of care: All patients received appropriate treatments for heart failure, including diuretics, inhibitors of the renin angiotensin system and neprilysin, beta blockers, mineralocorticoid receptor antagonists and, when indicated, cardiac devices.1
-
‡
Numbers shown are for the JARDIANCE arm.1
-
ACEi=angiotensin converting enzyme inhibitor; ARB=angiotensin II receptor blocker; ARNI=angiotensin receptor neprilysin inhibitor; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HHF= hospitalisation for heart failure; LVEF=left ventricular ejection fraction; MRA=mineralocorticoid receptor antagonist; NT-proBNP =N terminal pro-brain natriuretic peptide; NYHA=New York Heart Association; T2D=type 2 diabetes.
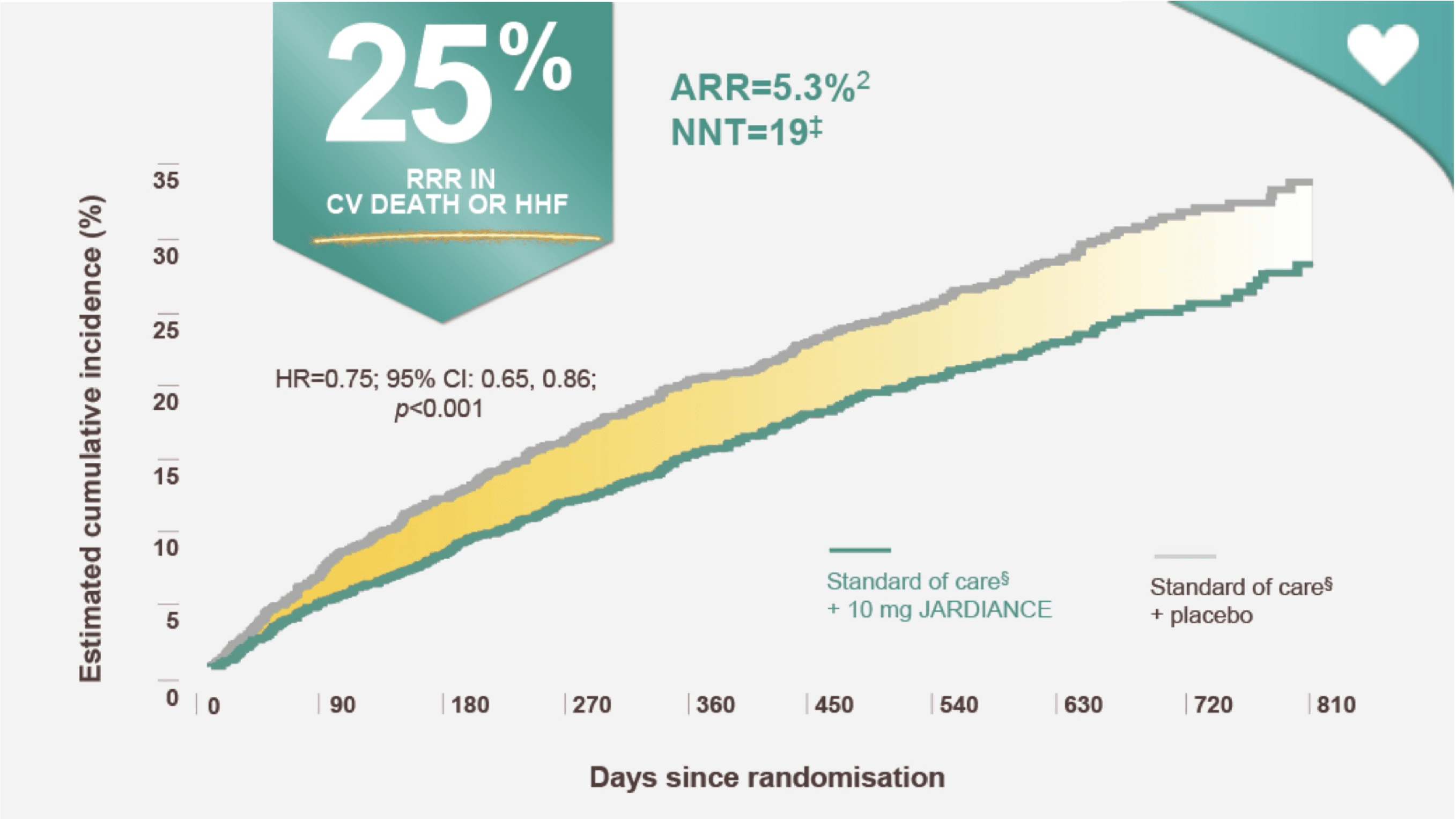
In the treatment of HF patients with LVEF ≤ 40%*
JARDIANCE reduced the risk of CV death or HHF†1
First and recurrent HHF eGFR slope
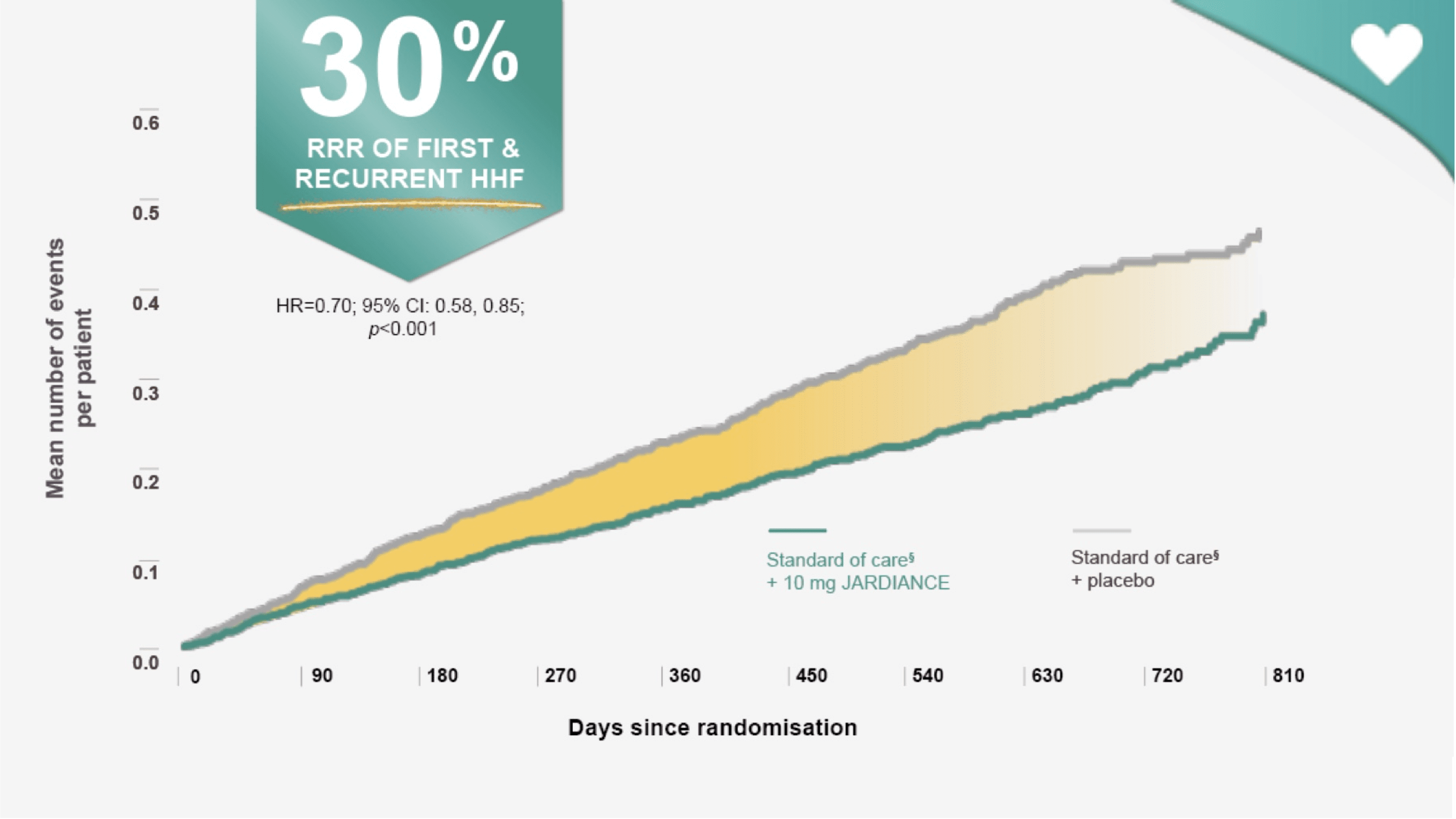
In the treatment of HF patients with LVEF ≤ 40%*
JARDIANCE reduced the risk of first and recurrent HHF||¶1
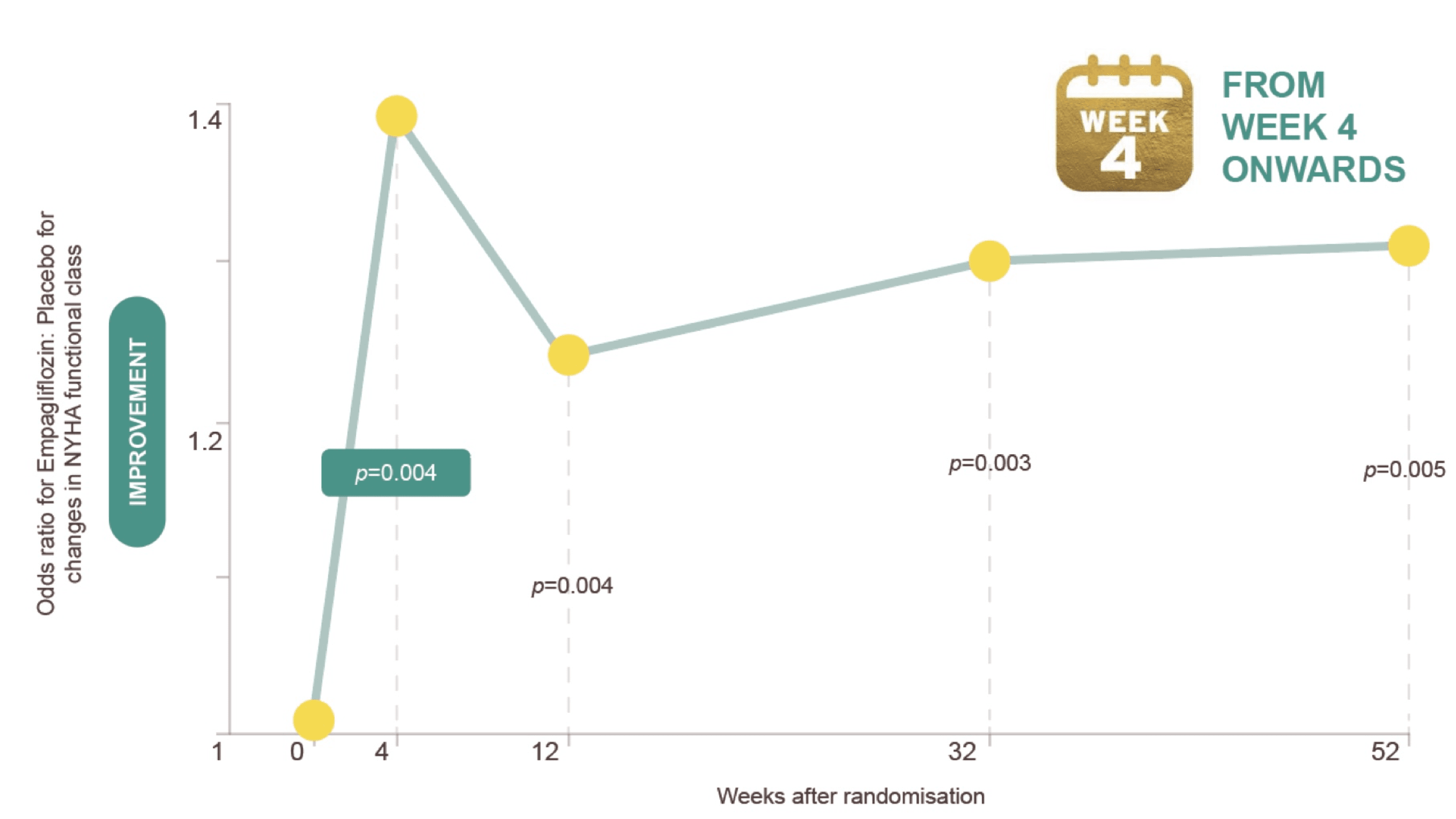
JARDIANCE® rapidly improves NYHA class in HFrEF patients3,4
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190 (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
Butler J, Zannad F, Filippatos G, Anker SD, Packer M. Ten lessons from the EMPEROR-Reduced trial. Eur J Heart Fail. 2020;22(11):1991-1993. doi:10.1002/ejhf.2009
-
McDonagh TA, et al. Eur Heart J. 2021;42:3599.
-
Packer M, et al. Circulation. 2021;143(4):326 336.
-
*
Adult patients with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤ 40%).1
-
†
In the EMPEROR Reduced trial, a randomised, double blind, parallel group, placebo controlled study of 3730 patients with HFrEF, the efficacy and safety of JARDIANCE 10 mg (n=1863) were evaluated vs placebo (n=1867). The primary endpoint in the EMPEROR-Reduced trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE experienced a 25% RRR in this endpoint (HR=0.75; 95% CI: 0.65,0.86; p<0.001).1
-
‡
ARR calculation: JARDIANCE number of patients with events 361/total number of patients 1863=19.4%; placebo number of patients with events 462/total number of patients 1867=24.7%; 24.7% 19.4%=5.3%.1,2
-
II
The occurrence of all HHF, including first and recurrent events, was a prespecified secondary outcome of the EMPEROR-Reduced trial.1
-
¶
In addition to the RRR of all HHF, including first and recurrent events, the risk of total hospitalisations of any reason was also significantly reduced (HR=0.85, 95% CI: 0.75, 0.95).1
-
§
Standard of care: All patients received appropriate treatments for heart failure, including diuretics, inhibitors of the renin-angiotensin system and neprilysin, beta blockers, mineralocorticoid receptor antagonists and, when indicated, cardiac devices.1
-
#
The rate of decline in eGFR was a prespecified secondary outcome of the EMPEROR-Reduced trial.1
-
**
Mean baseline eGFR (CKD EPI): JARDIANCE: 61.8 mL/min/1.73 m2; placebo: 62.2 mL/min/1.73 m2.1
-
ARNi=Angiotensin receptor neprilysin inhibitor; ARR=absolute risk reduction; CI=confidence interval; CKD EPI=Chronic Kidney Disease Epidemiology Collaboration; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HF=heart failure; HFrEF=heart failure with reduced ejection fraction; HHF= hospitalisation for heart failure; HR=hazard ratio; LVEF=left ventricular ejection fraction; NNT=number needed to treat; NYHA=New York Heart Association; RRR=relative risk reduction
PC-IN-103696 Validity till July 2025
