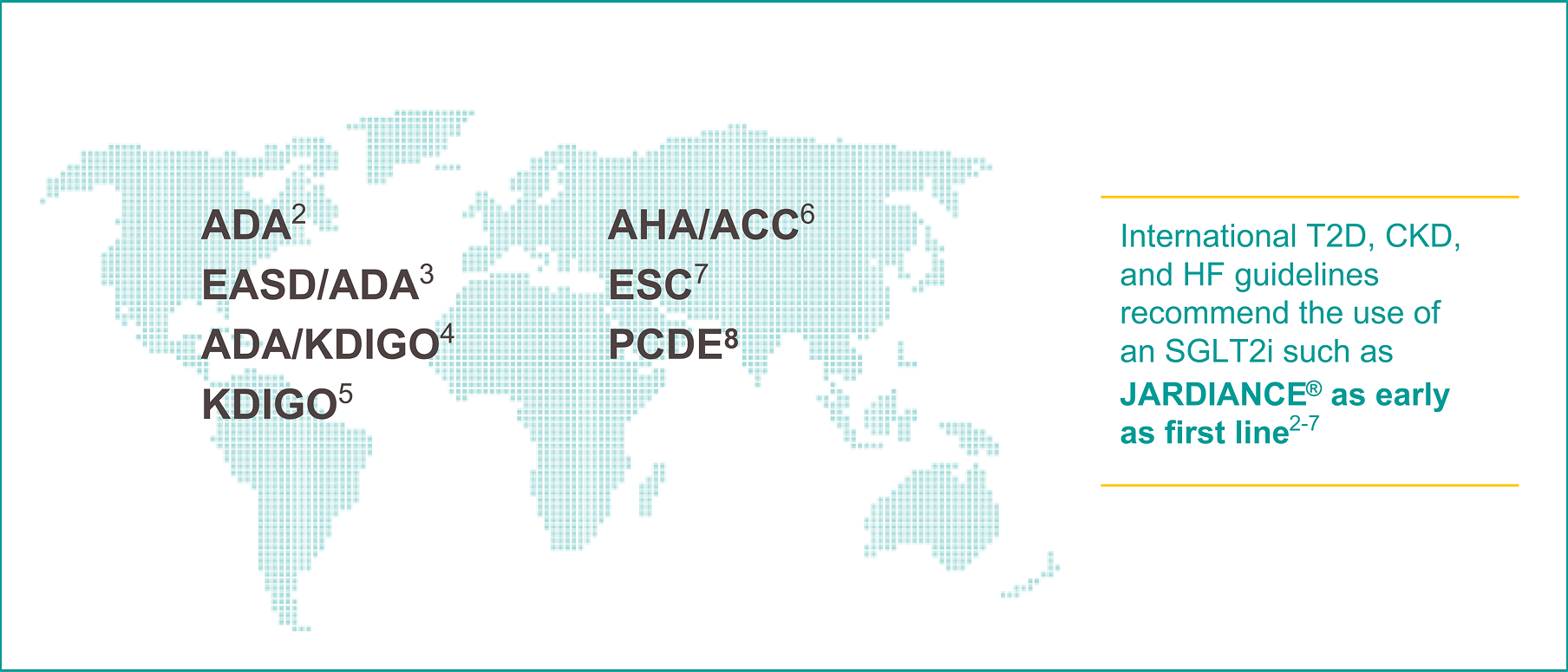
Guidelines
More than 100 endocrinology, nephrology, and cardiology guidelines, worldwide, endorse the foundational use of SGLT2is, such as JARDIANCE®1

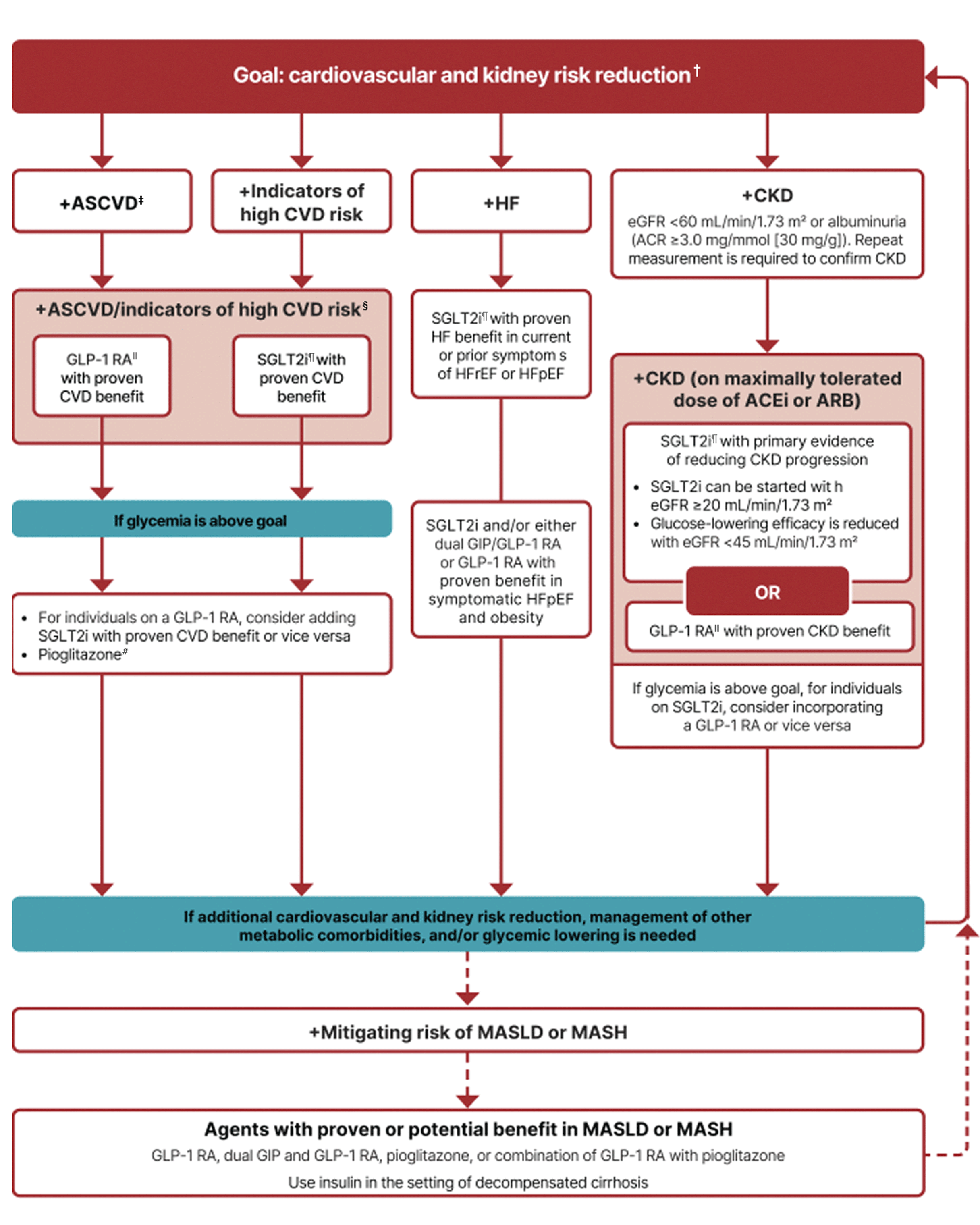
To reduce cardio-renal risk in high-risk patients with T2D, the 2025 ADA/EASD Consensus Report recommends*2
-
Data on file. Boehringer Ingelheim Pharmaceuticals, Inc.
-
American Diabetes Association Professional Practice Committee for Diabetes. 9. Pharmacologic approaches to glycemic treatment: Standards of Care in Diabetes—2026. Diabetes Care. 2026;49(Suppl. 1):S183–S215.
-
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786.
-
de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45(12):3075-3090.
-
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(suppl 4S):S117-S314.
-
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421.
-
McDonagh TA, Metra M, Adamo M, et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
-
Seidu S, Cos X, Brunton S, et al. 2022 update to the position statement by Primary Care Diabetes Europe: a disease state approach to the pharmacological management of type 2 diabetes in primary care. Prim Care Diabetes. 2022;16(2):223-244.
-
*
In people with HF, CKD, established CVD, or multiple risk factors for CVD, the decision to use a GLP-1 RA or SGLT2i with proven benefit should be made irrespective of attainment of glycemic goal.
-
†
ASCVD: Defined differently across CVOTs but all included individuals with established CVD (e.g., MI, stroke, and arterial revascularization procedure) and variably included conditions such as transient ischemic attack, unstable angina, amputation, and symptomatic or asymptomatic coronary artery disease. Indicators of high risk: While definitions vary, most comprise !55 years of age with two or more additional risk factors (including obesity, hypertension, smoking, dyslipidemia, or albuminuria).
-
$
A strong recommendation is warranted for people with CVD and a weaker recommendation for those with indicators of high risk CVD. Moreover, a higher absolute risk reduction and thus lower numbers needed to treat are seen at higher levels of baseline risk and should be factored into the shared decision-making process. See text for details.
-
#
For GLP-1 RAs, CVOTs demonstrate their efficacy in reducing composite MACE, CV death, all-cause mortality, MI, stroke, and kidney end points in individuals with T2D with established or high risk of CVD. One kidney outcome trial demonstrated benefit in reducing persistent eGFR reduction and CV death for a GLP-1 RA in individuals with CKD and T2D.
-
‡
For SGLT2is, CV and kidney outcomes trials demonstrate their efficacy in reducing the risks of composite MACE, CV death, all-cause mortality, MI, HHF, and kidney outcomes in individuals with T2D and established or high risk of CVD.
-
^
Low-dose pioglitazone may be better tolerated and similarly effective as higher doses.
-
ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin-to-creatinine ratio; ADA, American Diabetes Association; AHA, American Heart Association; ACC, American College of Cardiology; ARB, angiotensin receptor blocker; ASCVD, atherosclerotic cardiovascular disease; CGM, continuous glucose monitoring; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; CVOT, cardiovascular outcomes trial; DPP-4i, dipeptidyl peptidase-4 inhibitor; DSMES, diabetes self-management education and support; eGFR, estimated glomerular filtration rate; EASD, European Association for the Study of Diabetes; ESC, European Society of Cardiology; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; KDIGO, Kidney Disease: Improving Global Outcomes; MACE, major adverse cardiovascular events; MASH, metabolic dysfunction-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; MI, myocardial infarction; PCDE, Primary Care Diabetes Europe; SDOH, social determinants of health; SGLT2i, sodium–glucose cotransporter-2 inhibitor; T2D, type 2 diabetes.
PC-IN-103695
