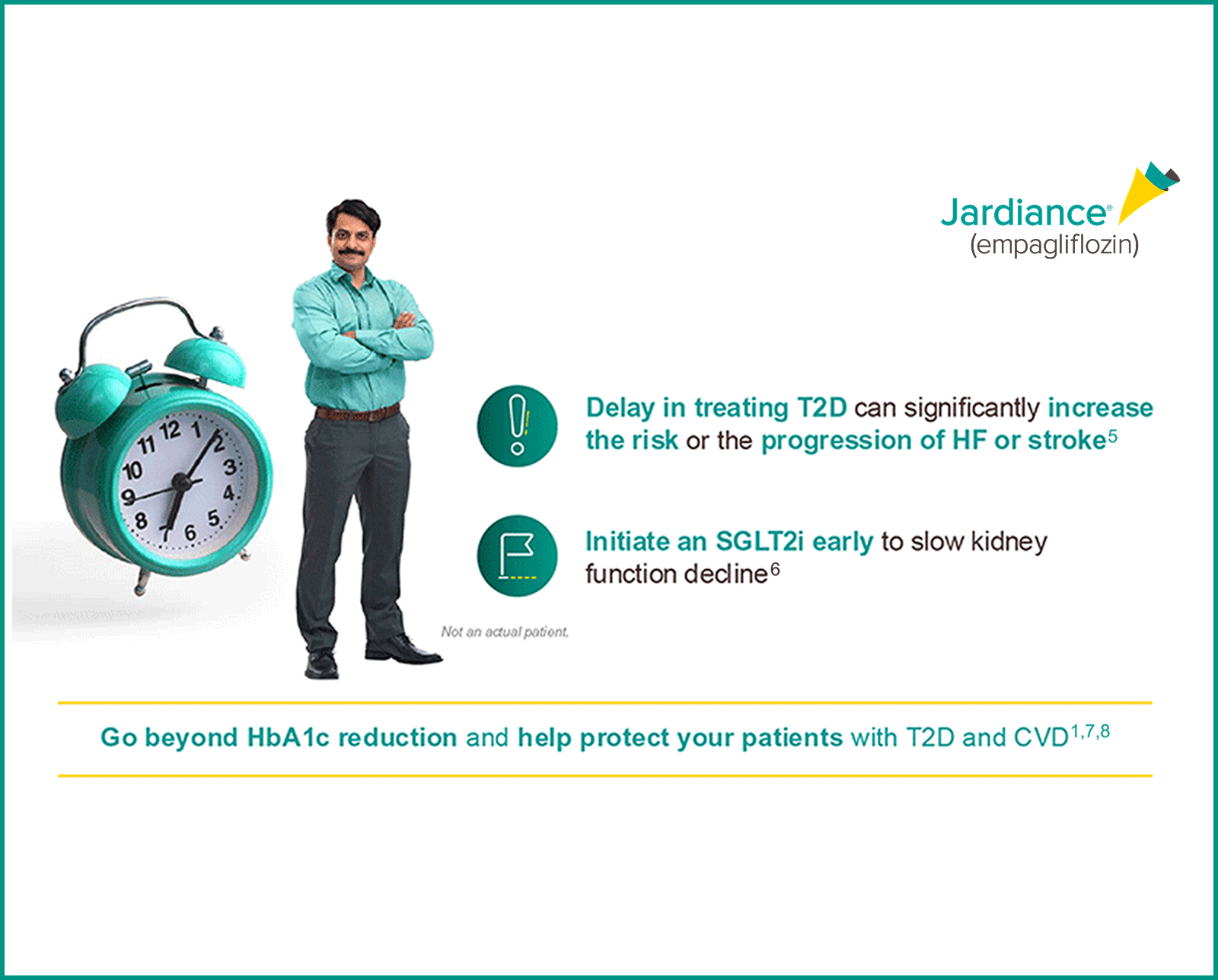
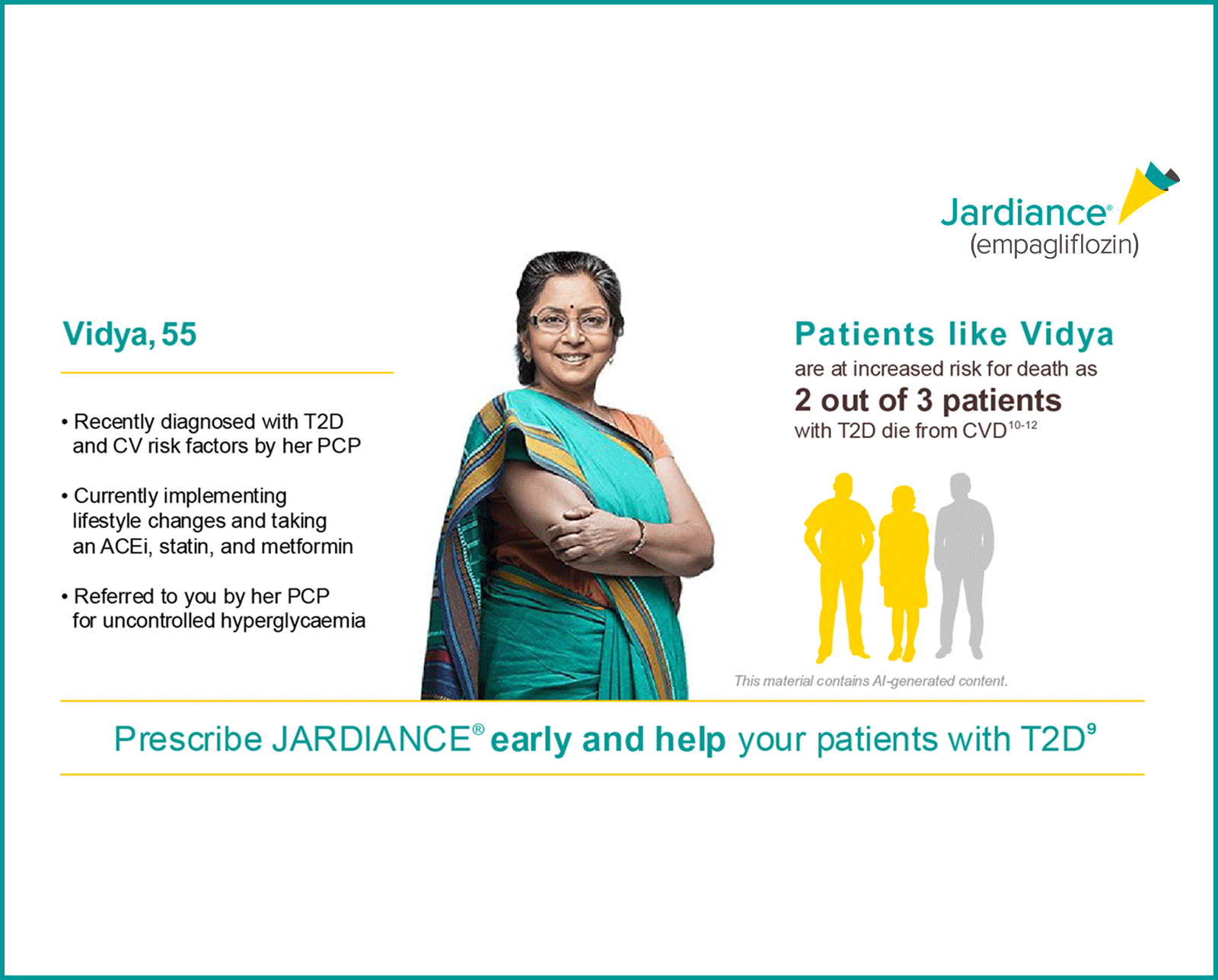
Why CRM?
For your patients with T2D,
JARDIANCE® provides multiple metabolic benefits to patients while also reducing CV and renal risk*9
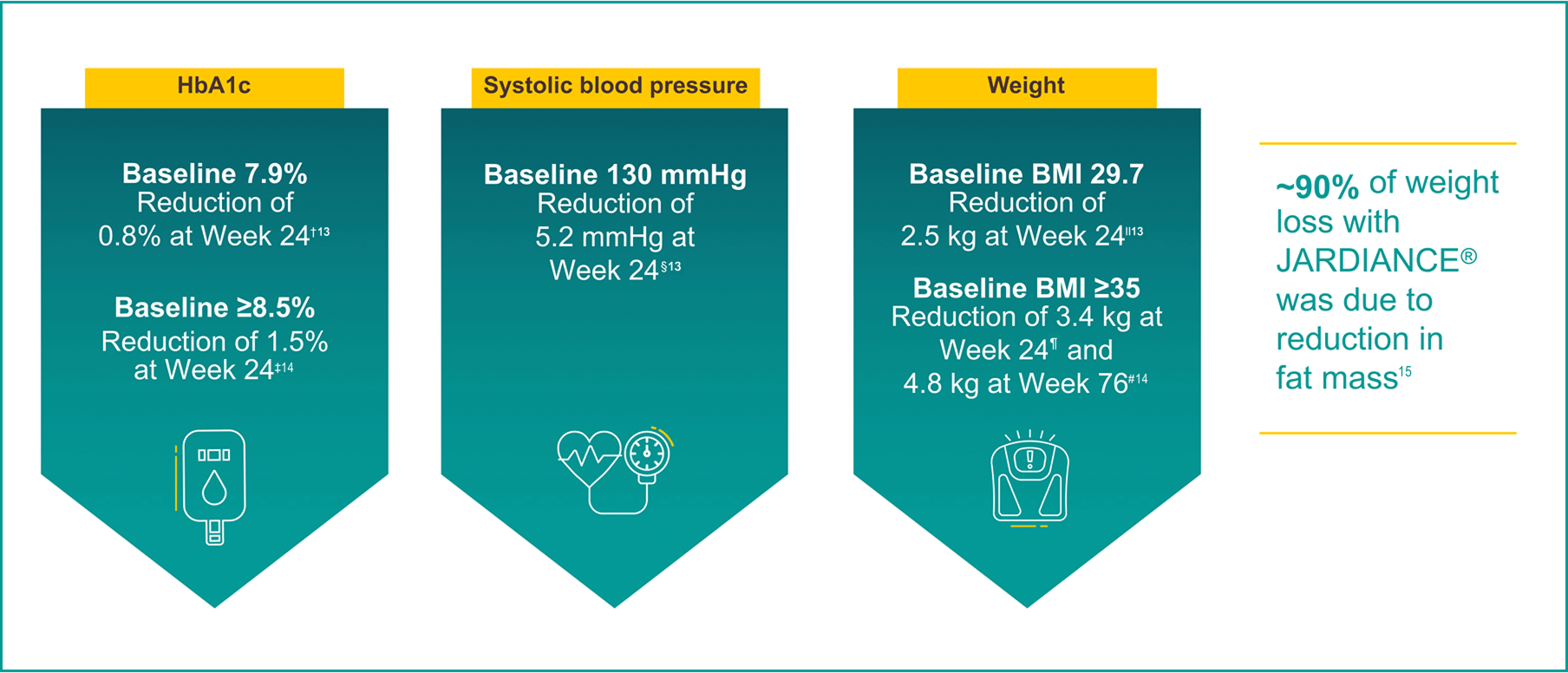
Reductions in body weight and systolic blood pressure were not primary endpoints.
Results from the EMPA-REG OUTCOME® trial.
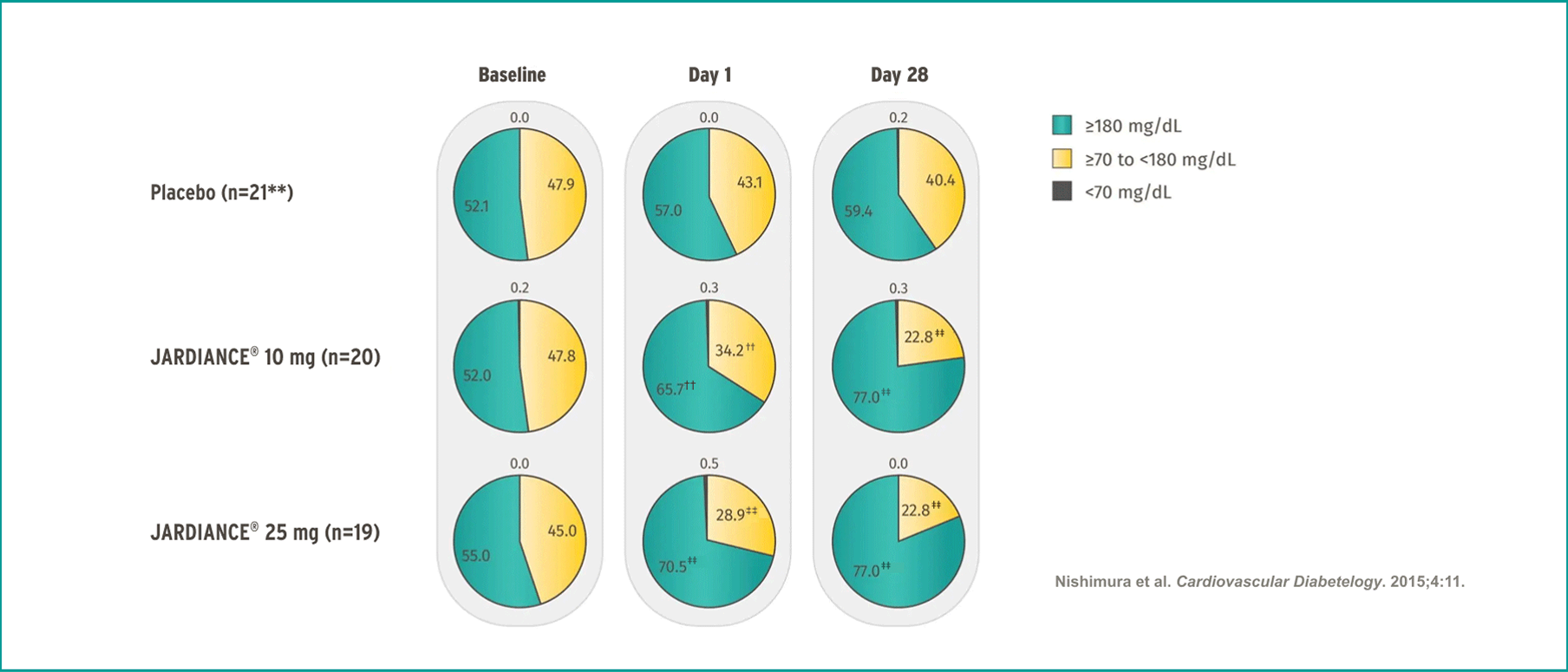
JARDIANCE® improves time-in-range in uncontrolled T2DM16
Benefit on 24-hr CGM is observed from day-1 onwards

In insulin-naïve patients with T2D and eCVD§§
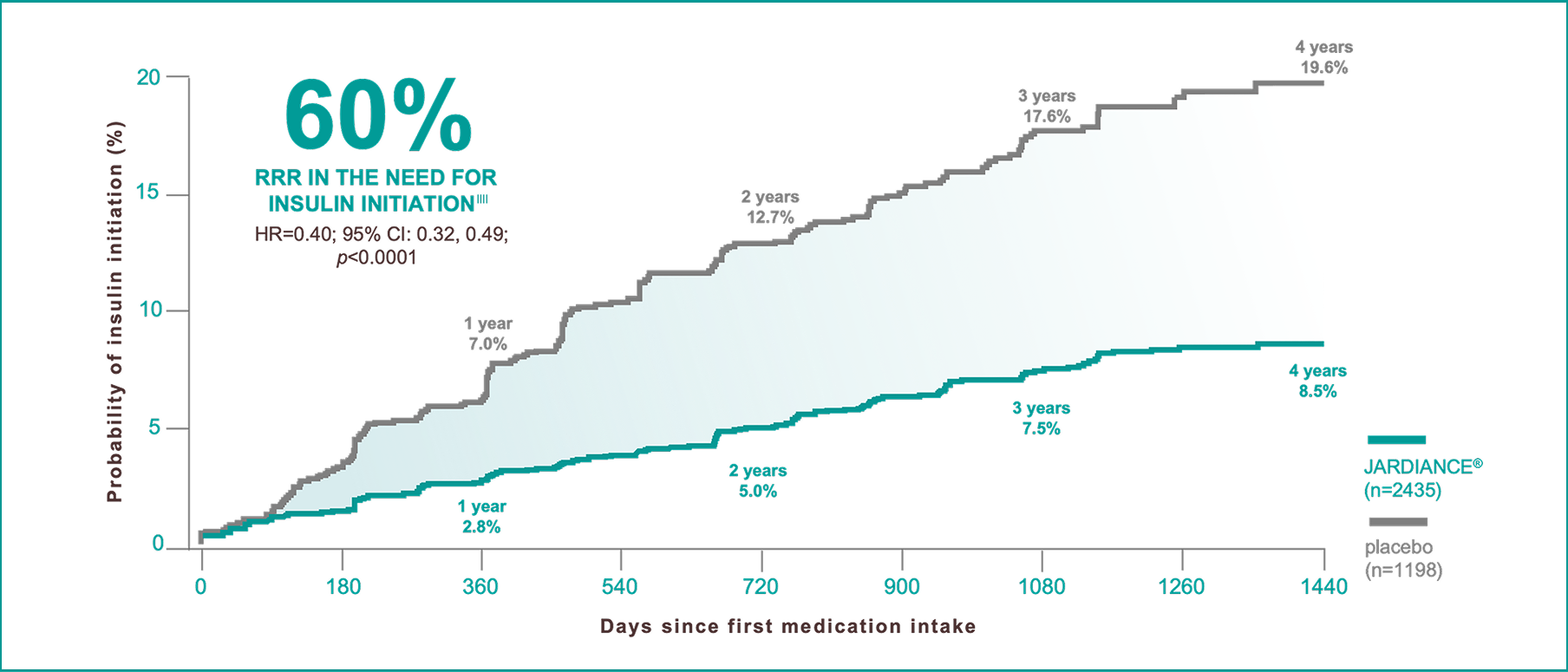
A post hoc analysis from the EMPA-REG OUTCOME® trial.

In insulin-treated patients with T2D and eCVD§§
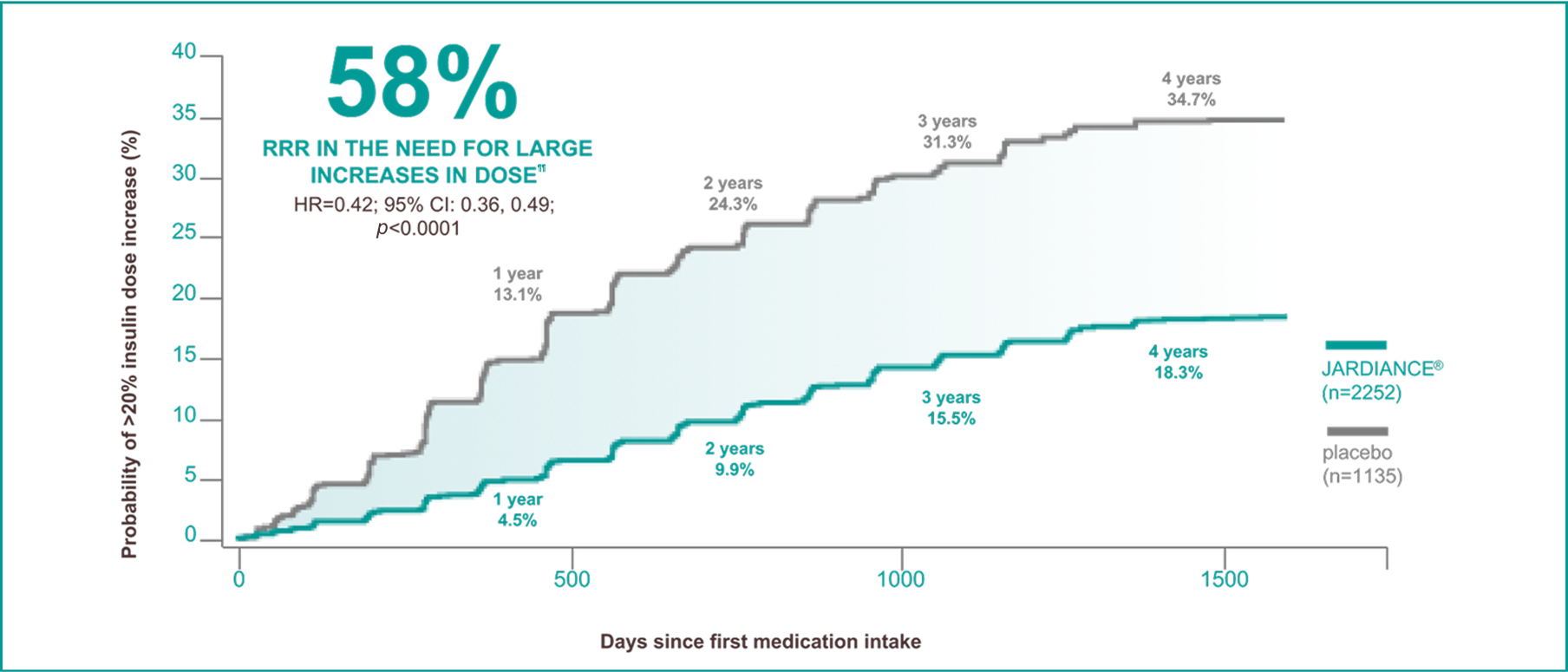
A post hoc analysis from the EMPA-REG OUTCOME® trial.
-
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753-2786.
-
McDonagh TA, Metra M, Adamo M, et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
-
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421.
-
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-733.
-
Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100.
-
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(suppl 4S):S117-S314.
-
Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes: implications for clinical practice. Prim Care. 1999;26(4):771-789.
-
UK Prospective Diabetes Study Group. UK prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes. 1995;44(11):1249-1258.
-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
-
Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms. Diabetes Care. 2010;33(2):442-449.
-
Ma C-X, Ma X-N, Guan C-H, Li Y-D, Mauricio D, Fu S-B. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21(74):1-15.
-
American Diabetes Association. Cardiovascular disease. Accessed September 25, 2024. https://diabetes.org/about-diabetes/complications/cardiovascular-disease.
-
Häring H-U, Merker L, Seewaldt-Becker E, et al; EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650-1659.
-
Inzucchi SE, Davies MJ, Khunti K, et al. Empagliflozin treatment effects across categories of baseline HbA1c, body weight, and blood pressure as an add-on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2021;23(2):425-433.
-
Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC; EMPA-REG H2H-SU Trial Investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691-700.
-
Nishimura R, Tanaka Y, Koiwai K, Inoue K, Hach T, Salsali A, Lund SS, Broedl UC. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:1-13.
-
Vaduganathan M, Inzucchi SE, Sattar N, et al. Effects of empagliflozin on insulin initiation or intensification in patients with type 2 diabetes and cardiovascular disease: findings from the EMPA-REG OUTCOME trial. Diabetes Obes Metab. 2021;23(12):2775-2784.
-
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix.)
-
*
In a 24-week, double-blind, placebo-controlled study of 637 patients with T2D, the efficacy and safety of JARDIANCE® 10 mg (n=217) and JARDIANCE® 25 mg (n=213) as add-on therapy to metformin ≥1500 mg were evaluated vs placebo added to metformin (n=207). The primary endpoint was adjusted mean change (SE) from baseline in HbA1c (%); weight loss and blood pressure reduction were key secondary and exploratory endpoints, respectively.13
-
†
Adjusted mean changes of -0.1% from baseline HbA1c 7.9% for placebo (n=207), -0.7% from baseline HbA1c 7.9% for JARDIANCE® 10 mg (n=217), and -0.8% from baseline HbA1c 7.9% for JARDIANCE® 25 mg (n=213), respectively. Difference from placebo (adjusted mean) was -0.6% for both JARDIANCE® 10 mg and 25 mg; p<0.001 vs placebo for both doses.13
-
‡
In a subgroup analysis at 24 weeks of patients with baseline ≥8.5%, adjusted mean changes in HbA1c were -0.5% for placebo (n=50), -1.2% for JARDIANCE® 10 mg (n=57), and -1.5% for JARDIANCE® 25 mg (n=48). Difference from placebo (adjusted mean) was -0.73% for JARDIANCE® 10 mg and -1.0% for JARDIANCE® 25 mg. Missing data were imputed using LOCF approach.14
-
§
Adjusted mean change in systolic blood pressure from baseline at 24 weeks: JARDIANCE® 10 mg -4.5 mmHg (n=217), JARDIANCE® 25 mg -5.2 mmHg (n=213), vs placebo -0.4 mmHg (n=207).13
-
||
Adjusted mean changes of -0.45 kg reduction in body weight from baseline 79.7 kg for placebo (n=207), -2.08 kg from baseline 81.6 kg for JARDIANCE® 10 mg (n=217), and -2.46 kg from baseline 82.2 kg for JARDIANCE® 25 mg (n=213), respectively; p<0.0001 vs placebo for both doses.13
-
¶
In a subgroup analysis at 24 weeks of patients with baseline BMI ≥35, adjusted mean changes in weight were -0.34 kg for placebo (n=29), -2.63 kg for JARDIANCE® 10 mg (n=33), and -3.35 kg for JARDIANCE® 25 mg (n=41). Difference from placebo (adjusted mean) was -2.28 for JARDIANCE® 10 mg and -3.01 for JARDIANCE® 25 mg.14
-
#
In a double-blind extension trial, adjusted mean changes in weight for patients with baseline BMI ≥35 at Week 76 were 0.23 kg for placebo (n=29), -3.74 kg for JARDIANCE® 10 mg (n=33), and -4.77 kg for JARDIANCE® 25 mg (n=41). Difference from placebo (adjusted mean) was -3.96 kg for JARDIANCE® 10 mg and -4.99 kg for JARDIANCE® 25 mg. Missing data were imputed using the LOCF approach.14
-
**
n=20 at day 28.
-
††
p<0.01 for difference vs placebo in change from baseline.
-
‡‡
p<0.00 for difference vs placebo in change from baseline.
-
§§
Adult patients with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke.18
-
||||
Outcome of insulin initiation was defined to be maintained on ≥2 consecutive visits ≥13 weeks apart. Cox regression model adjusted for baseline HbA1c, time since T2D diagnosis, BMI, eGFR, geographic region, and treatment status. Kaplan-Meier estimates.17
-
¶¶
Sustained insulin dose increase was defined as time to first increase in total daily insulin dose by >20% from baseline total daily insulin dose, sustained for at least 2 consecutive visits at a minimum of 13 weeks apart, analysed in patients treated with insulin at baseline.17
-
ACEi, angiotensin-converting enzyme inhibitor; BMI, body mass index; CGM, continuous glucose monitoring; CRM, cardio-renal-metabolic; CVD, cardiovascular disease; eCVD, established cardiovascular disease; HbA1c, glycated hemoglobin; HF, heart failure; LOCF, last observation carried forward; mmHg, millimeters of mercury; PCP, primary care physician; SE, standard error of the mean; SGLT2i, sodium-glucose cotransporter-2 inhibitors; T2D, type 2 diabetes; T2DM, type 2 diabetes mellitus.
PC-IN-103695