EMPEROR-Preserved trial results published in The New England Journal of Medicine in 20211
A randomised, double blind, parallel group, placebo controlled, event driven trial

patients with LVEF > 40%

countries

(median observation)

events observed
Prespecified efficacy endpoints
-
Composite primary endpoint: Time to first event of adjudicated CV death or adjudicated HHF
-
Confirmatory secondary endpoints: Total number of HHF, including first and recurrent; rate of decline in eGFR from baseline
Key inclusion criteria
-
Chronic heart failure (NYHA class II-IV)
-
Elevated NT proBNP and structural heart changes or documented HHF within 12 months*
-
Anker SD, Butler J, Filippatos G, et al; EMPEROR Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. doi:10.1056/NEJMoa2107038 (EMPEROR-Preserved results and the publication's Supplementary Appendix.)
-
*
Defined as a level of > 300 pg /mL or, for patients with atrial fibrillation at baseline, a level of > 900 pg /mL.1
-
†
Background therapy: All appropriate treatments for heart failure or comorbid conditions could be initiated or altered at
the discretion of the clinician.1 -
‡
Numbers shown are for the JARDIANCE arm.1
-
ACEi=angiotensin converting enzyme inhibitor; ARB=angiotensin II receptor blocker; ARNI=angiotensin receptor neprilysin inhibitor; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HHF= hospitalisation for heart failure; LVEF=left ventricular ejection fraction; MRA=mineralocorticoid receptor antagonist; NT proBNP =N terminal pro brain natriuretic peptide; NYHA=New York Heart Association; T2D=type 2 diabetes.
EMPEROR Preserved trial results published in The New England Journal of Medicine in 20211
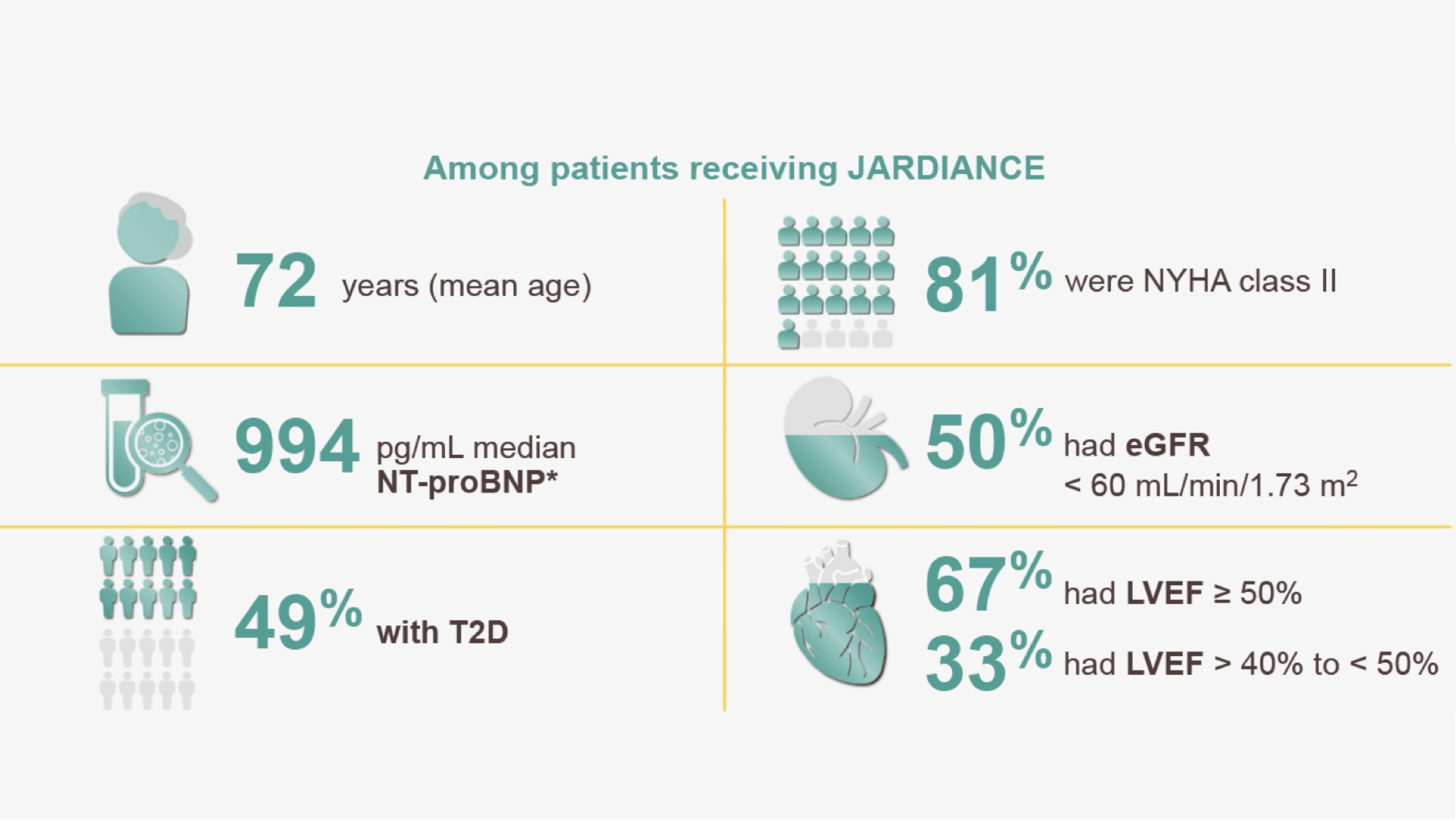
Patient characteristics

-
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. doi:10.1056/NEJMoa2107038 (EMPEROR-Preserved results and the publication's Supplementary Appendix.)
-
*
Defined as a level of > 300 pg/mL or, for patients with atrial fibrillation at baseline, a level of >900 pg/mL.1
-
†
Background therapy: All appropriate treatments for heart failure or comorbid conditions could be initiated or altered at
the discretion of the clinician.1 -
‡
Numbers shown are for the JARDIANCE arm.1
-
ACEi=angiotensin converting enzyme inhibitor; ARB=angiotensin II receptor blocker; ARNI=angiotensin receptor neprilysin inhibitor; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HHF=hospitalisation for heart failure; LVEF=left ventricular ejection fraction; MRA=mineralocorticoid receptor antagonist; NT-proBNP=N terminal pro-brain natriuretic peptide; NYHA=New York Heart Association; T2D=type 2 diabetes.
In the treatment of HF patients with LVEF > 40%*
JARDIANCE is the 1st medicine clinically proven to reduce the risk of CV death or HHF‡1
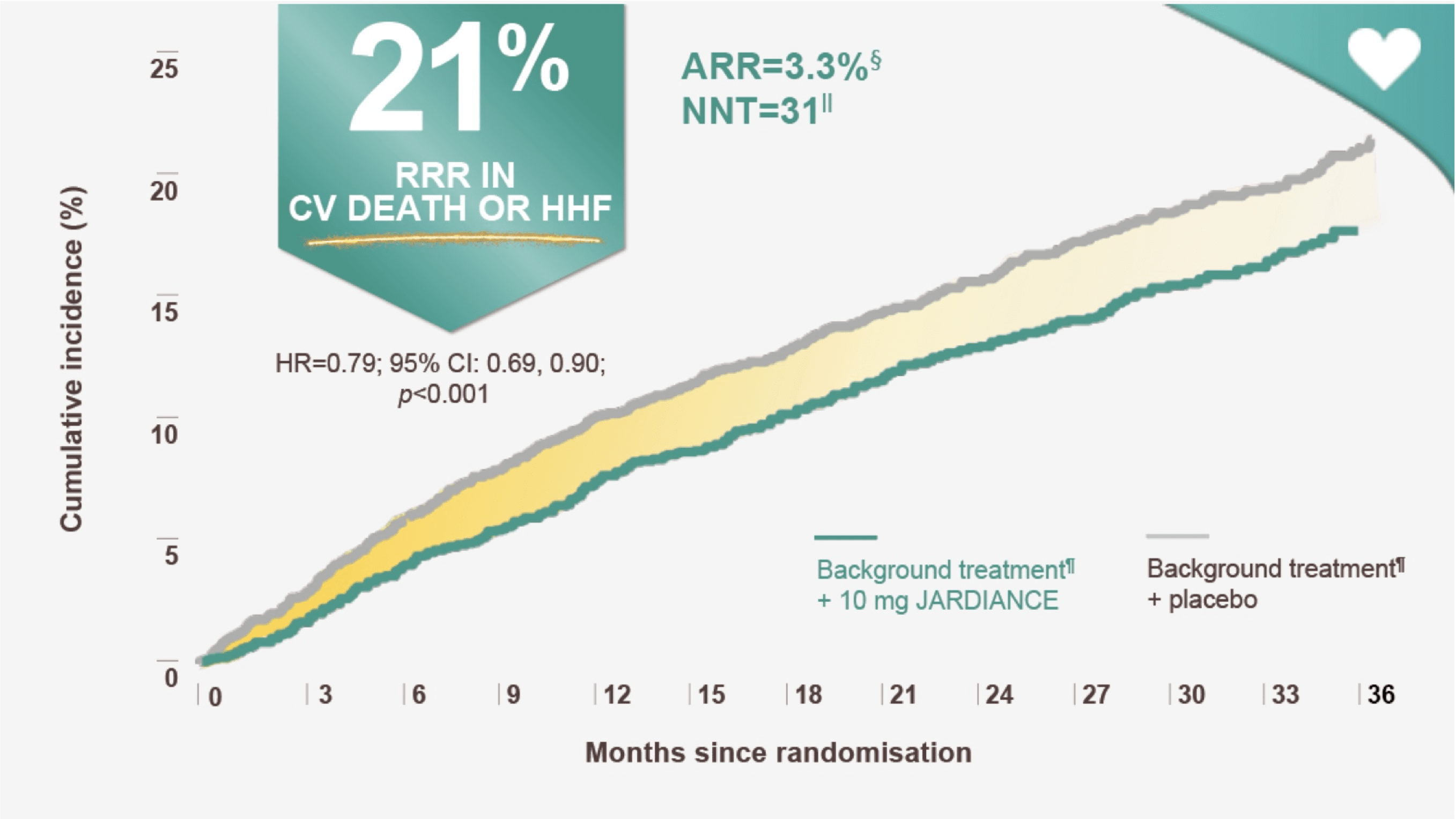
†Proven=meeting the primary endpoint in clinical trials.
Consistent efficacy across LVEF subgroups and T2D or CKD status1
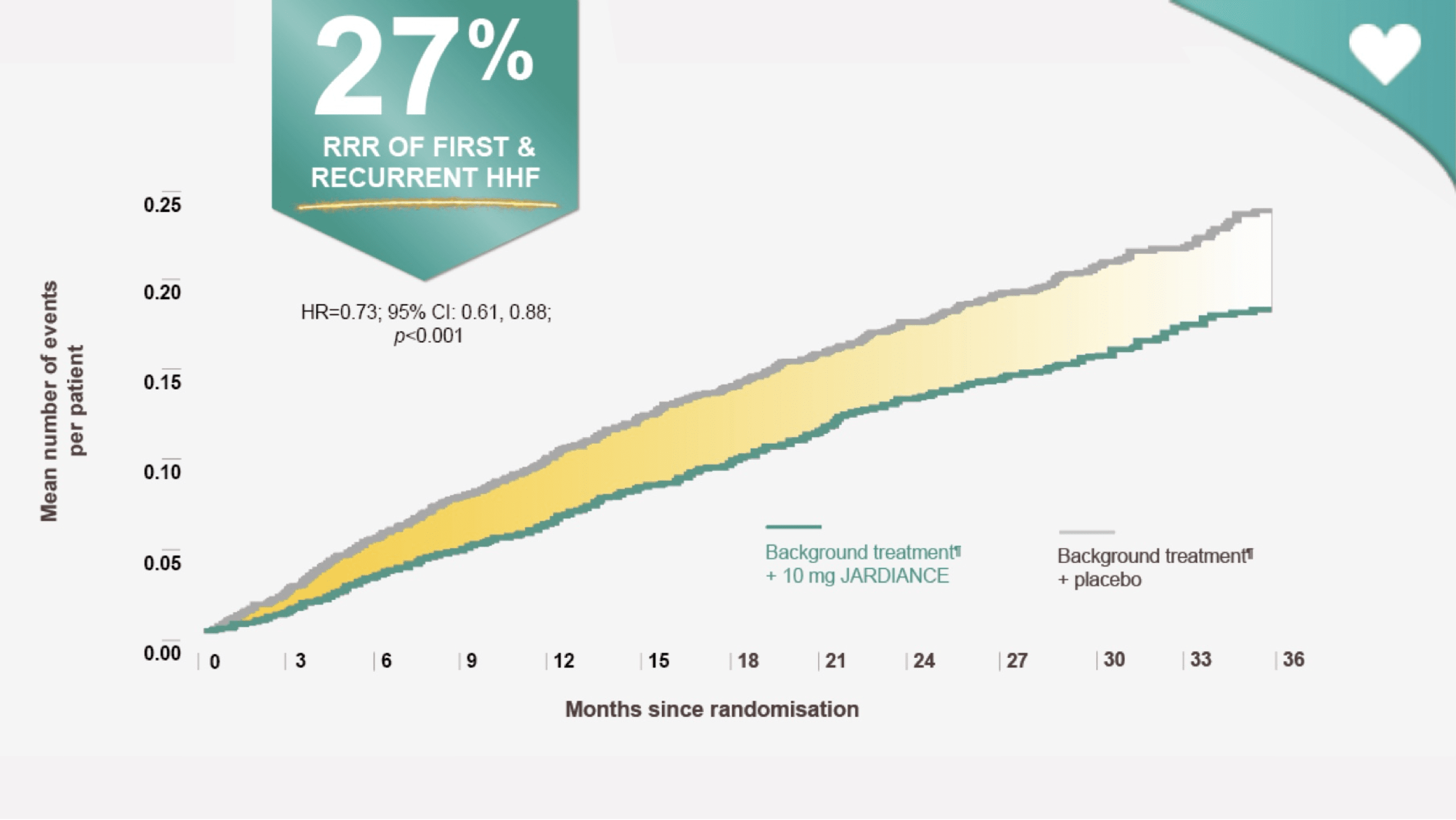
First and recurrent HHF
In the treatment of HF patients with LVEF > 40%*
JARDIANCE reduced the risk of first and recurrent HHF#1
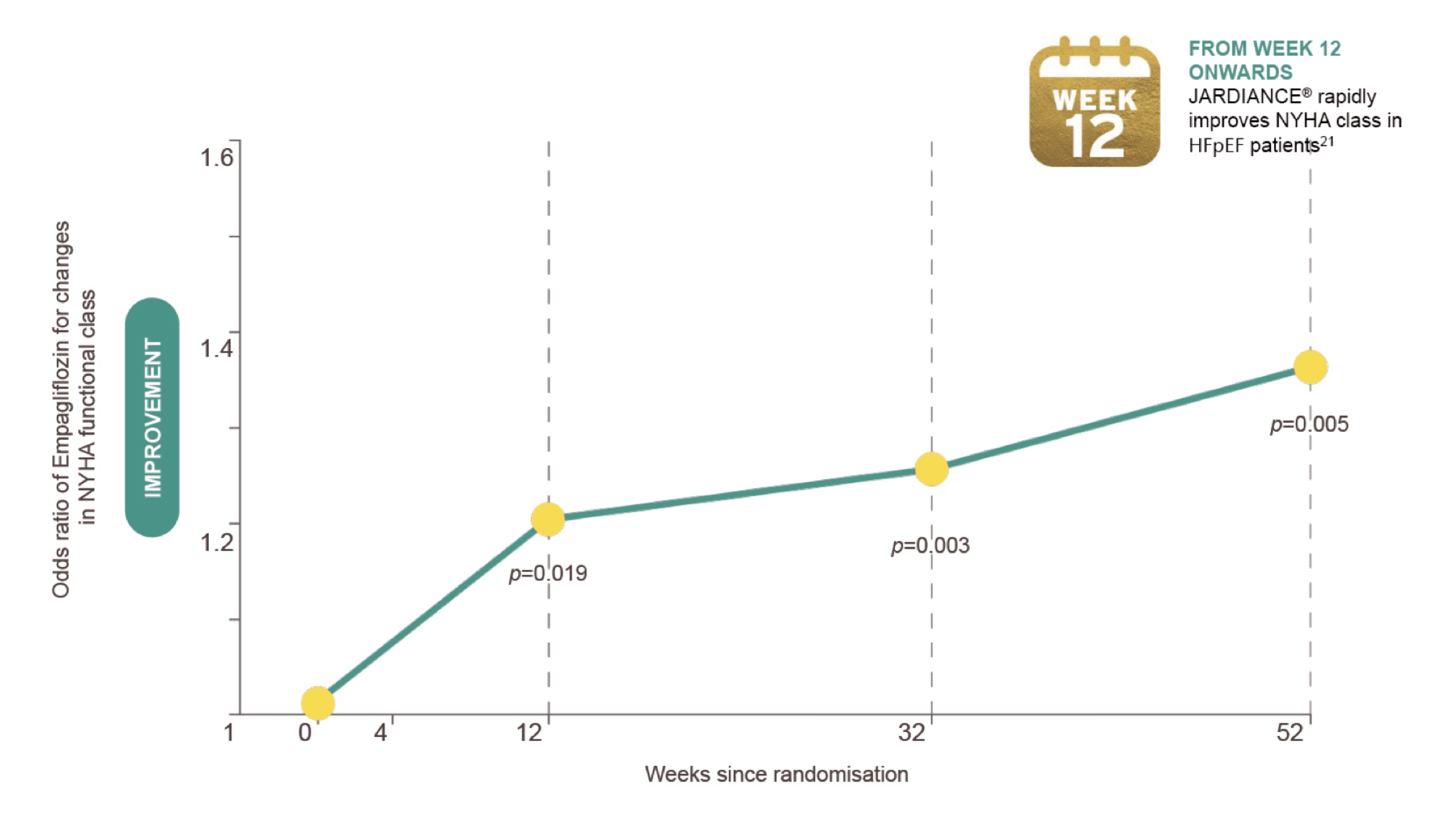
In patients with HFpEF Jardiance® provides early onset of symptomatic relief (NYHA class improvement)21
-
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. doi:10.1056/NEJMoa2107038 (EMPEROR-Preserved results and the publication’s Supplementary Appendix.)
-
Butler J, Siddiqi TJ, Filippatos G, et al. Early benefit with empagliflozin in heart failure with preserved ejection
fraction: insights from the EMPEROR-Preserved trial. Eur J Heart Fail. Published online January 5, 2022.
doi:10.1002/ejhf.2420
-
*
Adult patients with chronic heart failure (NYHA class II, III, or IV) and preserved ejection fraction (LVEF > 40%).1
-
‡
In the EMPEROR-Preserved trial, a randomised , double blind, parallel-group, placebo-controlled study of 5988 patients with HFpEF, the efficacy and safety of JARDIANCE 10 mg (n=2997) were evaluated vs placebo (n=2991). The primary endpoint in the EMPEROR-Preserved trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE experienced a 21% RRR in this endpoint (HR=0.79; 95% CI: 0.69, 0.90; p<0.001).1
-
§
ARR calculation: JARDIANCE number of patients with events 415/total number of patients 2997=13.8%; placebo number of patients with events 511/total number of patients 2991=17.1%; 17.1% 13.8%=3.3%.1
-
||
ARR was estimated as the absolute difference in the proportion of events by treatment arm. NNT=1/ARR.1
-
¶
Background treatment: All appropriate treatments for heart failure or comorbid conditions could be initiated or altered at the discretion of the clinician.1
-
#
The occurrence of all HHF, including first and recurrent events, was a prespecified secondary outcome of the
EMPEROR-Preserved trial.1 -
ARR=absolute risk reduction; CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; HF=heart failure;
HFpEF =Heart failure with preserved ejection fraction; HHF=hospitalisation for heart failure; HR=hazard ratio; LVEF=left ventricular ejection fraction; NNT=number needed to treat; NYHA=New York Heart Association; RRR=relative risk reduction; T2D=type 2 diabetes.
PC-IN-103696 Validity till July 2025
