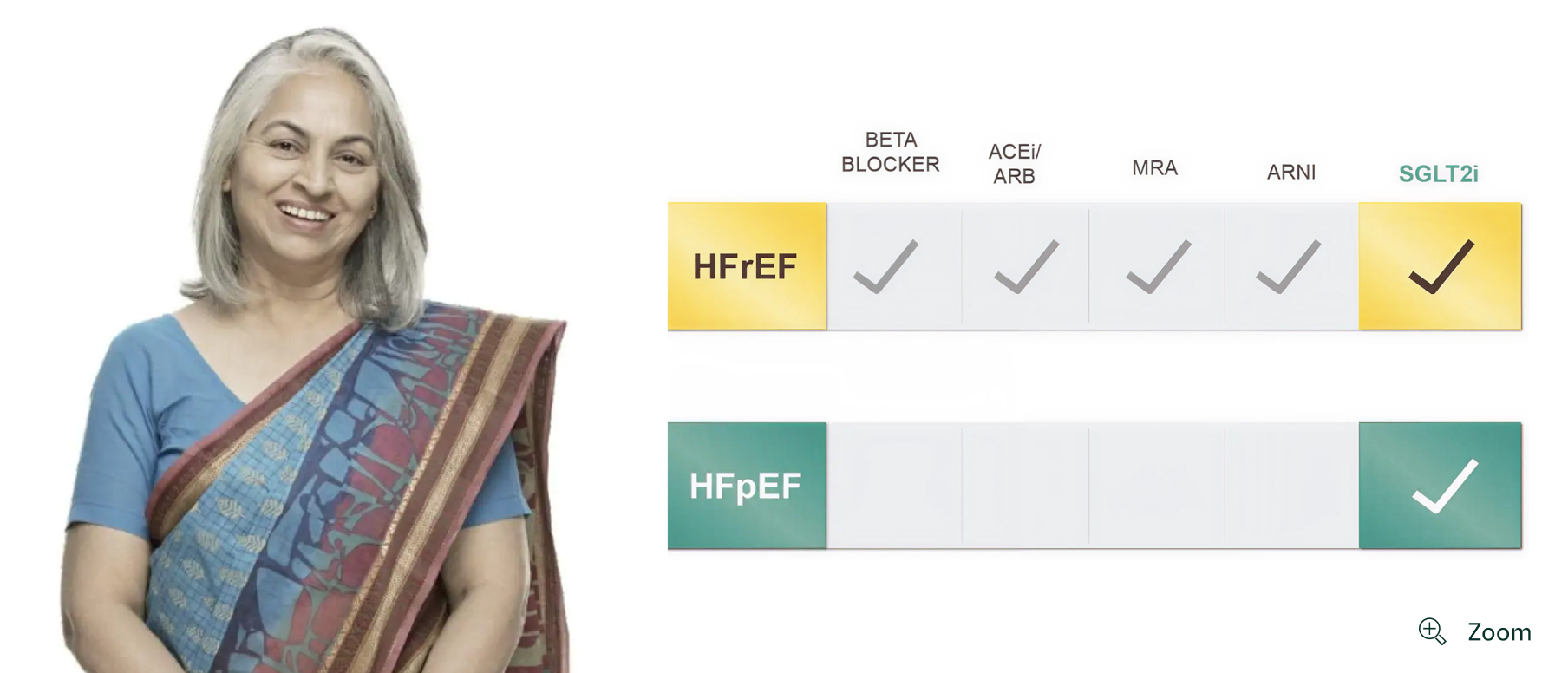
Patient profile

Palak, 66
Referred to you by her PCP for cardiac review

Not an actual patient.
This material contains AI-generated content.

HFpEF

- Hypercholesterolaemia
- Dyspnoea
- Oedema
- NYHA class II, progressing to III

- Statin
45

55%-60%

140/88

Add JARDIANCE early to reduce her risk of CV death or HHF
-
*
Adult patients with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤40%).1-3
-
†
Adult patients with chronic heart failure (NYHA class II, III, or IV) and preserved ejection fraction (LVEF >40%).2
-
CV=cardiovascular; eGFR=estimated glomerular filtration rate; HF=heart failure; HFpEF=heart failure with preserved ejection fraction; HFrEF=heart failure with reduced ejection fraction; HHF=hospitalisation for heart failure; LVEF=left ventricular ejection fraction; NYHA=New York Heart Association; PCP=primary care physician.
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. (EMPEROR-Preserved results and the publication’s Supplementary Appendix.)
-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
Until now, treatment in HFpEF has been focused on reducing symptoms and managing comorbidities1
And patients with HFpEF still experience:

CV DEATH

HOSPITALISATION

-
CV=cardiovascular; HFpEF=heart failure with preserved ejection fraction; HHF=hospitalisation for heart failure.
-
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726. doi:10.1093/eurheartj/ehab368
-
Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476-2486. doi:10.1016/j.jacc.2017.08.074
-
Khan MS, Sreenivasan J, Lateef N, et al. Trends in 30-and 90-day readmission rates for heart failure. Circ Heart Fail. 2021;14(4):e008335. doi:10.1161/CIRCHEARTFAILURE.121.008335
JARDIANCE is the first clinically proven and approved therapy to treat HFpEF1-10
Treatments proven*† to reduce the risk of CV death or HHF
Antihypertensives are effective in certain comorbidities. Diuretics are effective at reducing symptoms of congestion.
* Proven=meeting the primary endpoint in clinical trials.
-
†
Adult patients with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤ 40%).
-
Adult patients with chronic heart failure (NYHA class II, III, or IV) and preserved ejection fraction (LVEF > 40%).11,12
-
ACEi=angiotensin-converting enzyme inhibitor; ARB=angiotensin II receptor blocker; ARNI=angiotensin receptor neprilysin inhibitor; CV=cardiovascular; HFpEF=heart failure with preserved ejection fraction; HFrEF=heart failure with reduced ejection fraction; HHF=hospitalisation for heart failure; LVEF=left ventricular ejection fraction; MRA=mineralocorticoid receptor antagonist; NYHA=New York Heart Association.
-
Granger CB, McMurray JJV, Yusuf S, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362(9386):772-776. doi:10.1016/S0140-6736(03)14284-5
-
Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet. 2003;362(9386):777-781. doi:10.1016/S0140-6736(03)14285-7
-
Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN; SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302. doi:10.1056/NEJM199108013250501
-
Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J; PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338-2345. doi:10.1093/eurheartj/ehl250
-
Massie BM, Carson PE, McMurray JJ, et al; I-PRESERVE Investigators. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456-2467. doi:10.1056/NEJMoa0805450
-
Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11-21. doi:10.1056/NEJMoa1009492
-
Pitt B, Zannad F, Remme WJ, et al; Randomised Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709-717. doi:10.1056/NEJM199909023411001
-
Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383-1392. doi:10.1056/NEJMoa1313731
-
McMurray JJV, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi:10.1056/NEJMoa1409077
-
Solomon SD, McMurray JJV, Anand IS, et al; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609-1620. doi:10.1056/NEJMoa1908655
-
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190 (EMPEROR-Reduced results and the publication’s Supplementary Appendix.)
-
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. doi:10.1056/NEJMoa2107038 (EMPEROR-Preserved results and the publication's Supplementary Appendix.)
In the treatment of patients with HF with LVEF >40%*
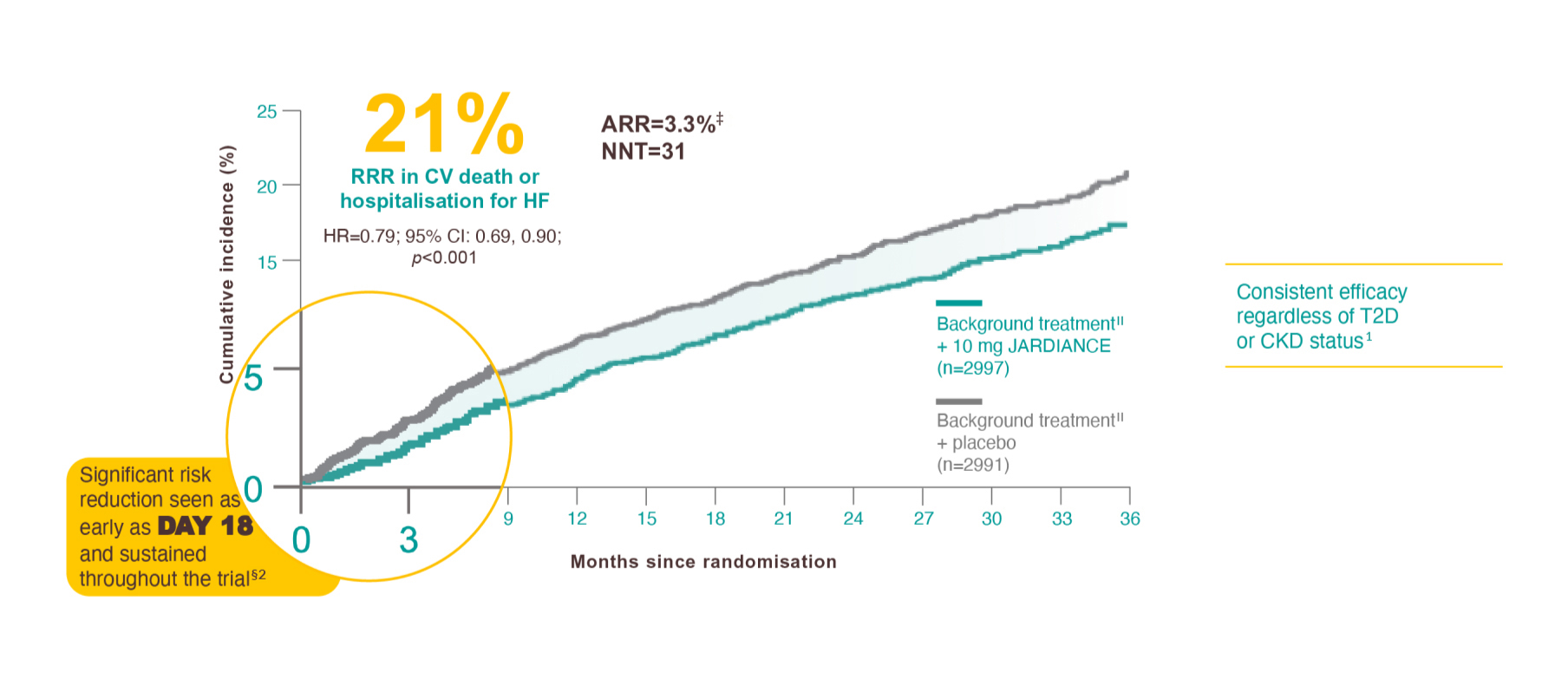
Results from the EMPEROR-Preserved trial.

In the treatment of patients with HF with LVEF ≤40%*
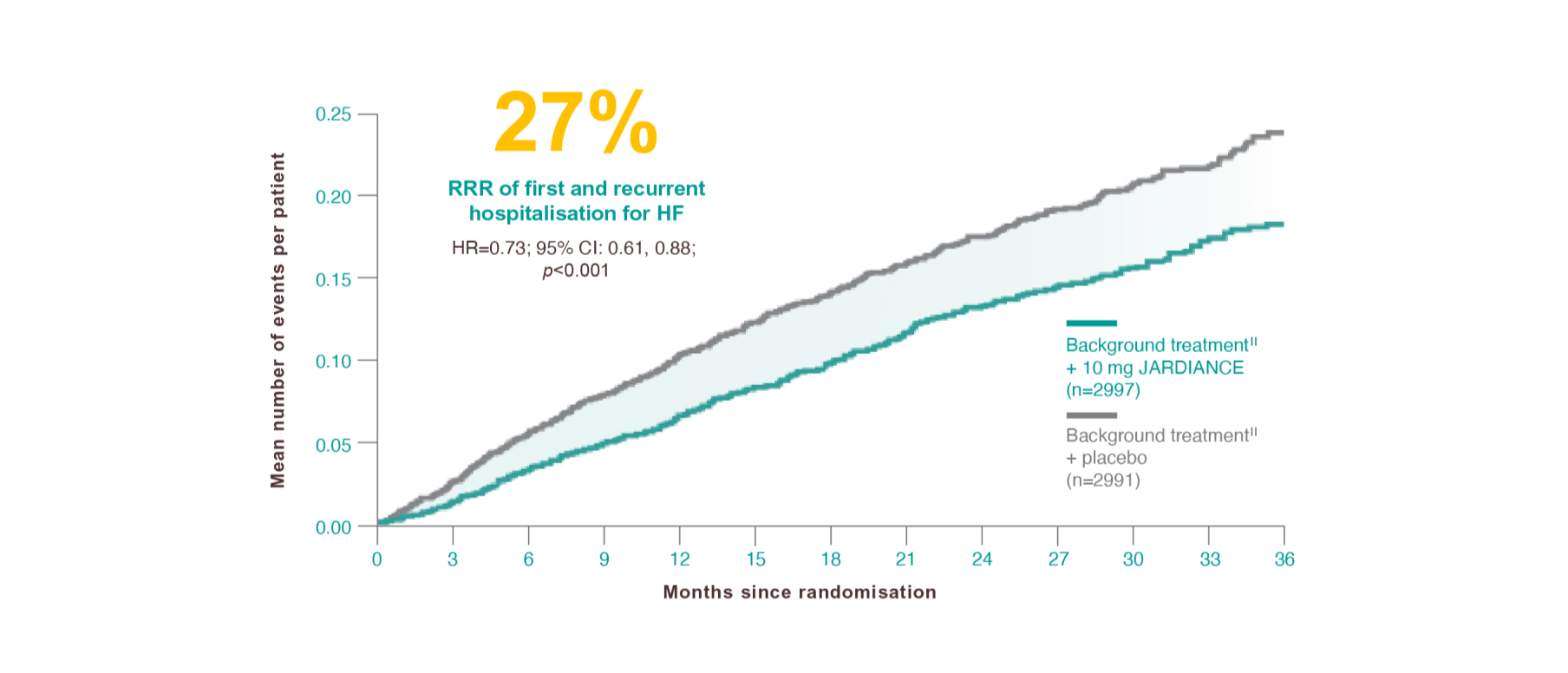
Results from the EMPEROR-Preserved trial.
-
*
Adult patients with chronic heart failure (NYHA class II, III, or IV) and reduced ejection fraction (LVEF ≤40%).1
-
†
In the EMPEROR-Preserved trial, a randomised, double-blind, parallel-group, placebo-controlled study of 5988 patients with HFpEF, the efficacy and safety of JARDIANCE 10 mg (n=2997) were evaluated vs placebo (n=2991). The primary endpoint in the EMPEROR-Preserved trial was a composite of CV death or HHF, analysed as time to the first event. Patients treated with JARDIANCE experienced a 21% RRR in this endpoint (HR=0.79; 95% CI: 0.69, 0.90; p<0.001).1
-
‡
ARR calculation: JARDIANCE number of patients with events 415/total number of patients 2997=13.8%; placebo number of patients with events 511/total number of patients 2991=17.1%; 17.1%–13.8%=3.3%. NNT=1/ARR.1
-
§
Reduction in the risk of CV death or HHF.2
-
II
Background treatment: All appropriate treatments for heart failure or comorbid conditions could be initiated or altered at the discretion of the clinician.1
-
¶
The occurrence of all HHF, including first and recurrent events, was a prespecified secondary outcome of the EMPEROR-Preserved trial.1
-
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. (EMPEROR-Preserved results and the publication’s Supplementary Appendix.)
-
Butler J, Siddiqi TJ, Filippatos G, et al. Early benefit with empagliflozin in heart failure with preserved ejection fraction: insights from the EMPEROR-Preserved Trial. Eur J Heart Fail. 2022;24(2):245-248.
In the treatment of patients with LVEF > 40%*
JARDIANCE has a proven safety and tolerability profile1
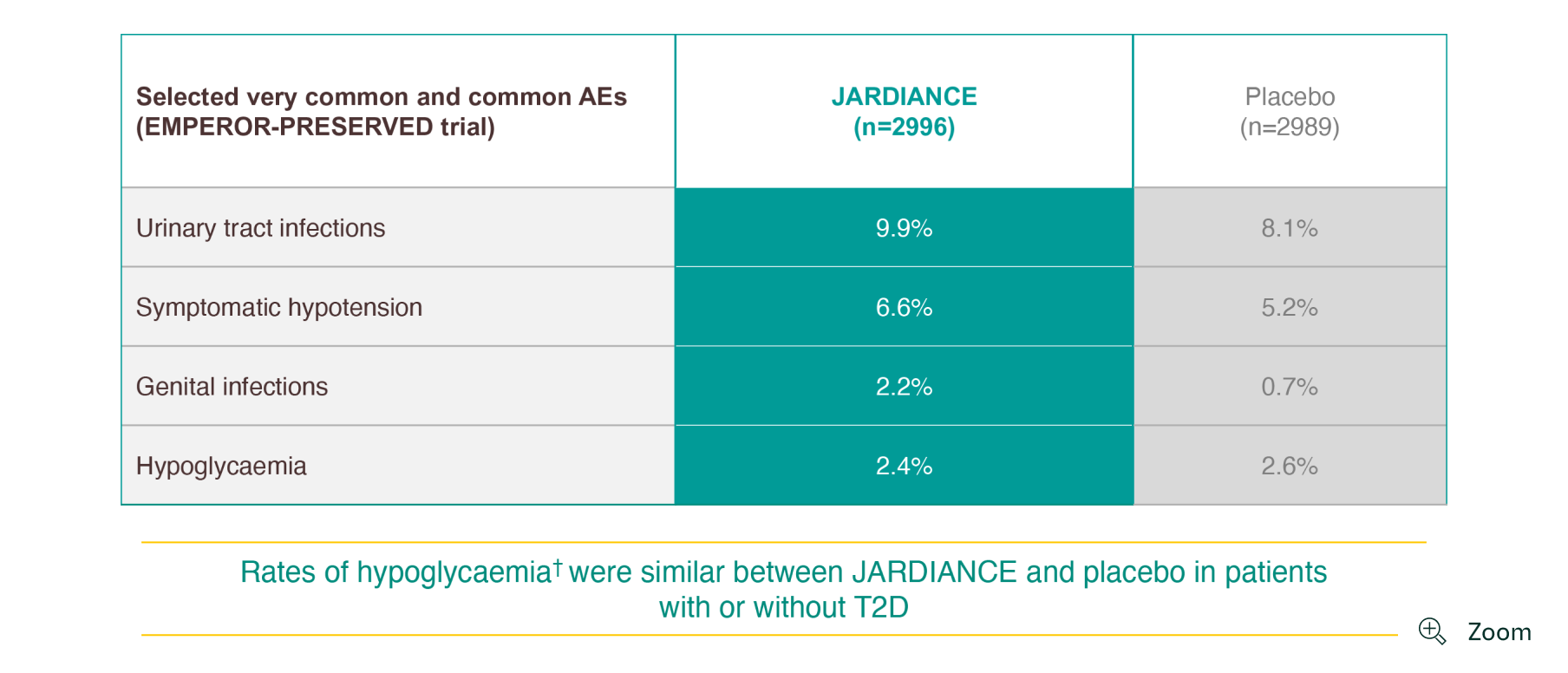
-
*
Adult patients with chronic HF (NYHA class II, III, or IV) and preserved ejection fraction (LVEF >40%).1
-
†
Hypoglycaemic AEs with a plasma glucose value of ≤70 mg/dL (3.9 mmol/L) or that required assistance.1
-
AE=adverse event; HF=heart failure; LVEF=left ventricular ejection fraction; NYHA=New York Heart Association; T2D=type 2 diabetes
-
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. (EMPEROR-Preserved results and the publication’s Supplementary Appendix.)
