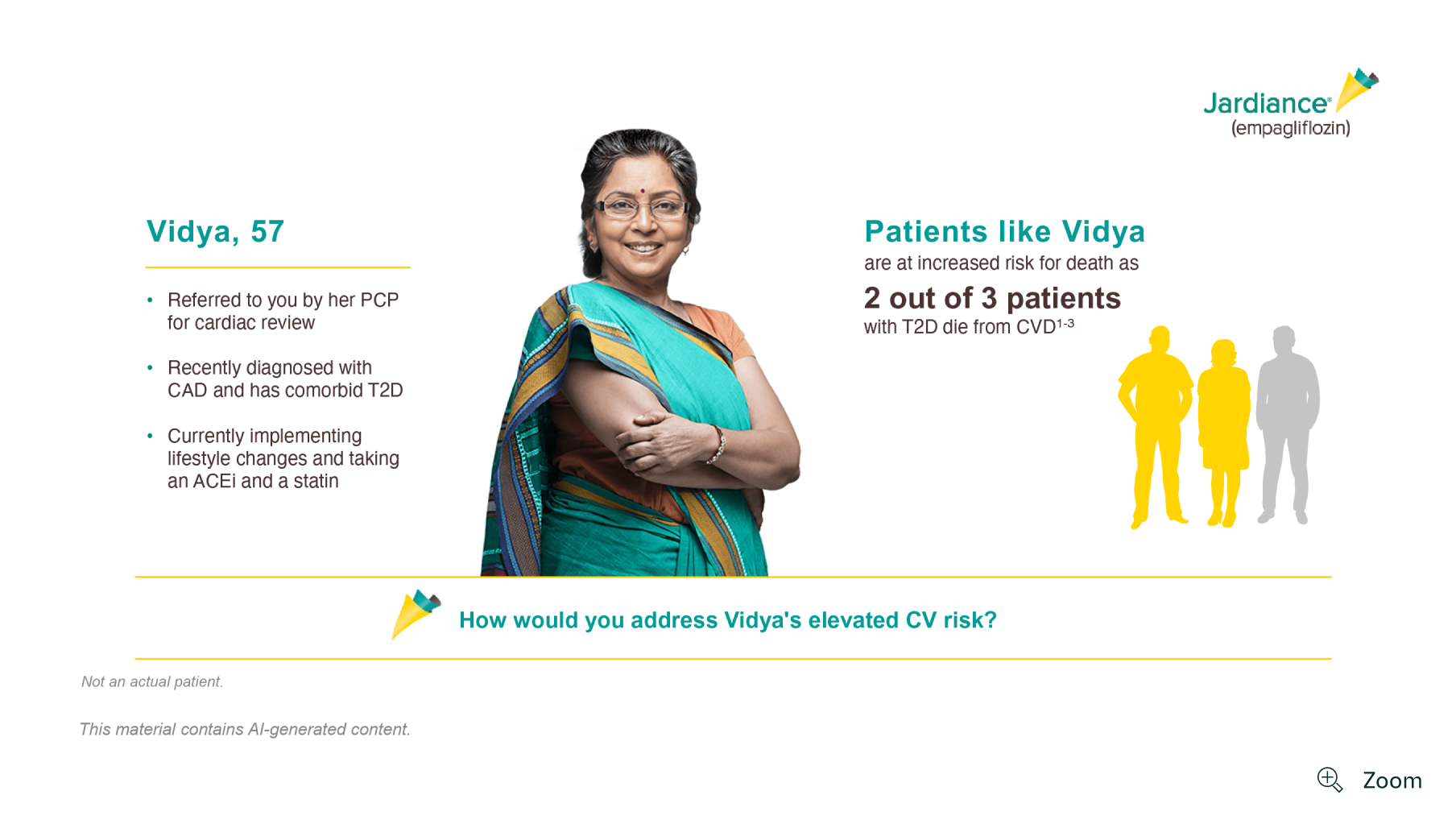
Patient profile

Vidya, 57
Referred to you by her PCP for cardiac review

Not an actual patient.
This material contains AI-generated content.

CAD and T2D

Uncontrolled hypertension, hypercholesterolaemia, overweight (BMI 29)

Lifestyle changes, ACEi, statin, metformin
86

Not tested

133/84
8%

Treatment optimisation is needed to reach targets
- Perform CV risk assessment including family history
- Start JARDIANCE
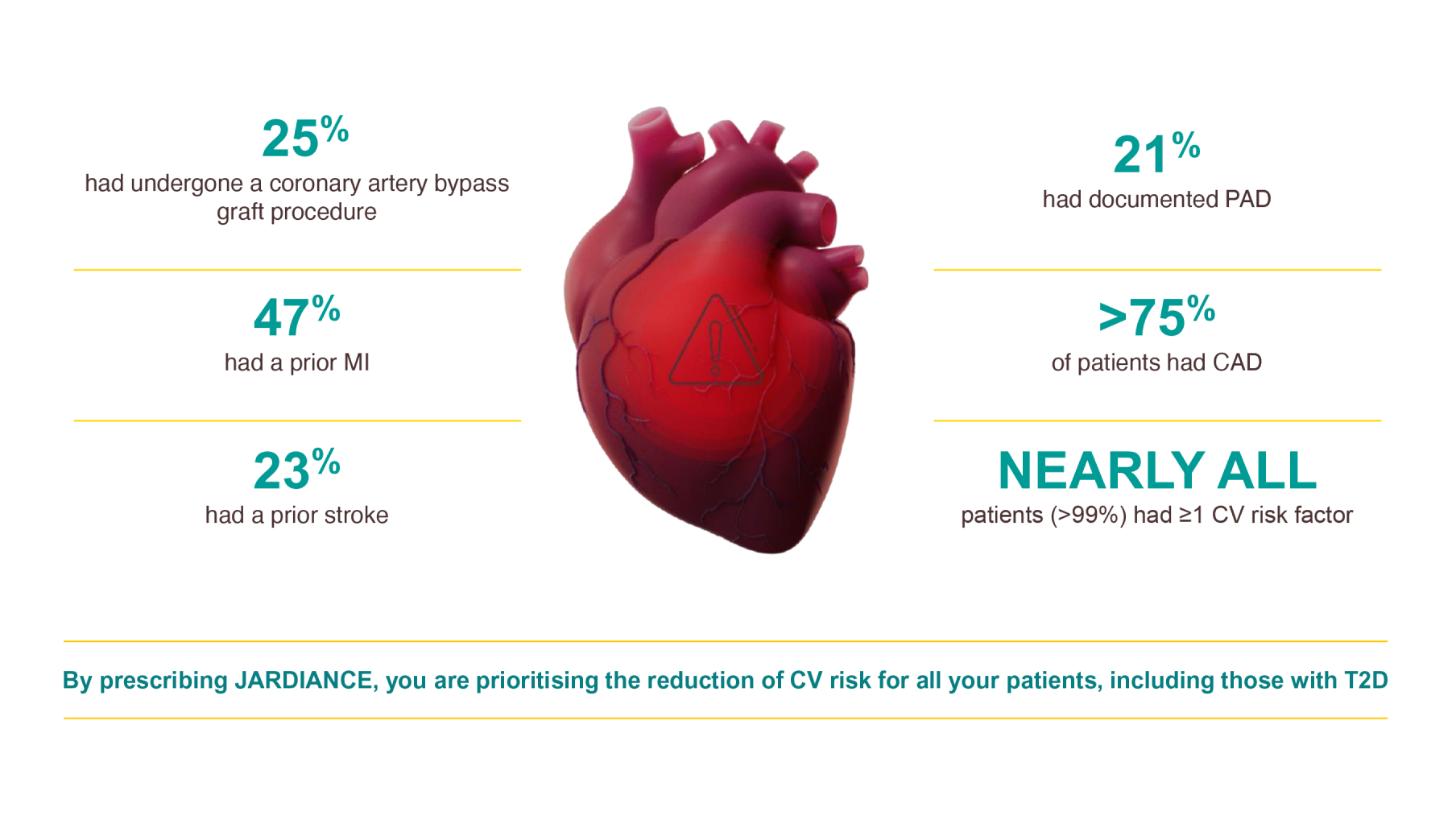
Patients with CAD and T2D may be referred to you to address their elevated CV risk
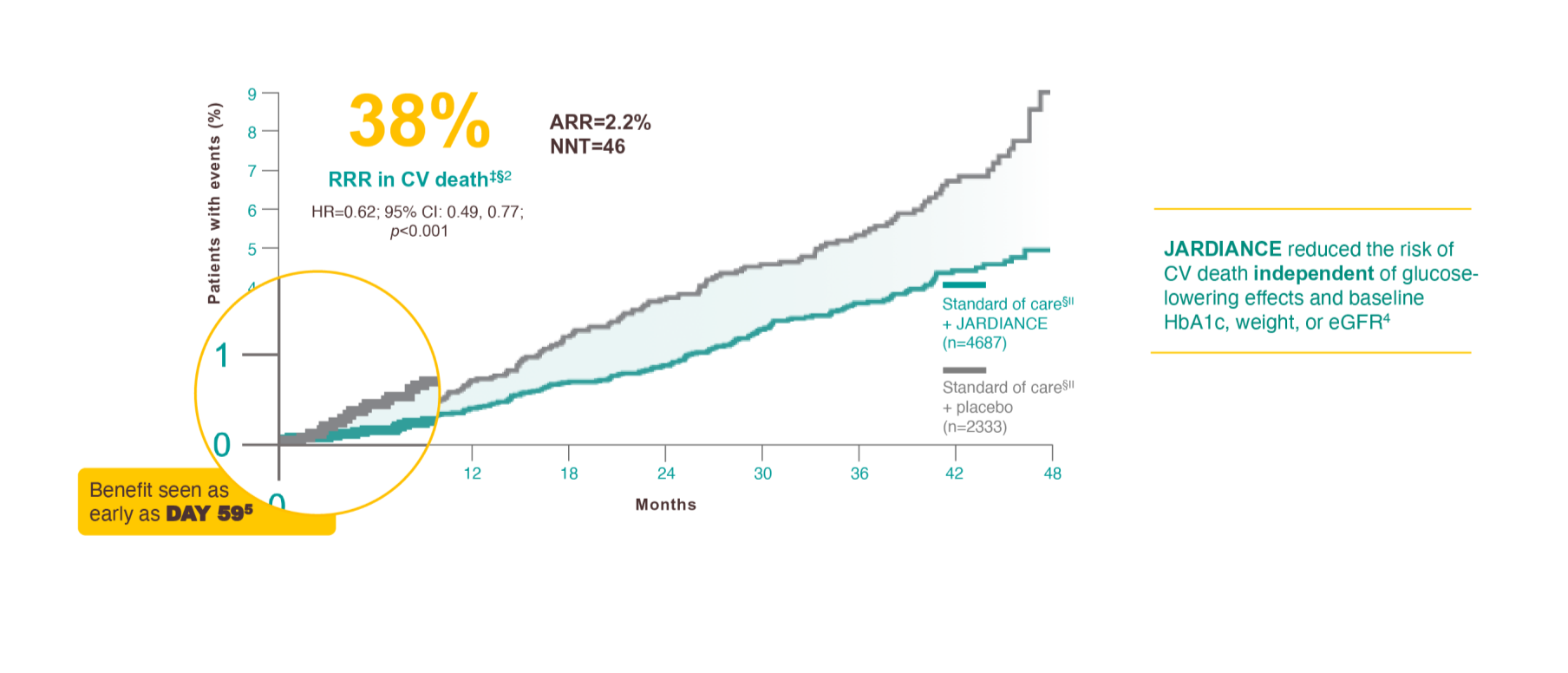
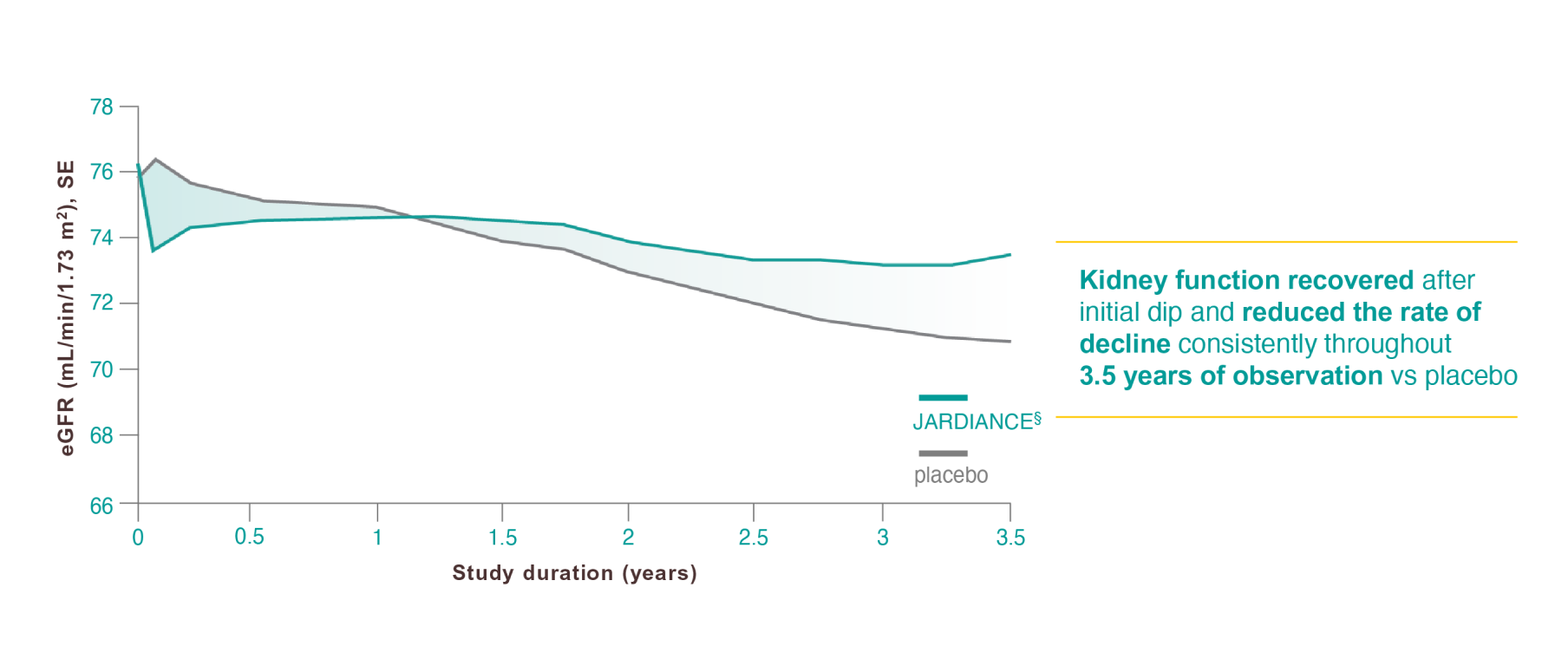
Results from the EMPA-REG OUTCOME® trial.
JARDIANCE is the only SGLT2i proven to reduce the risk of CV death in patients with eCVD + T2D*†
Results from the EMPA-REG OUTCOME® trial.
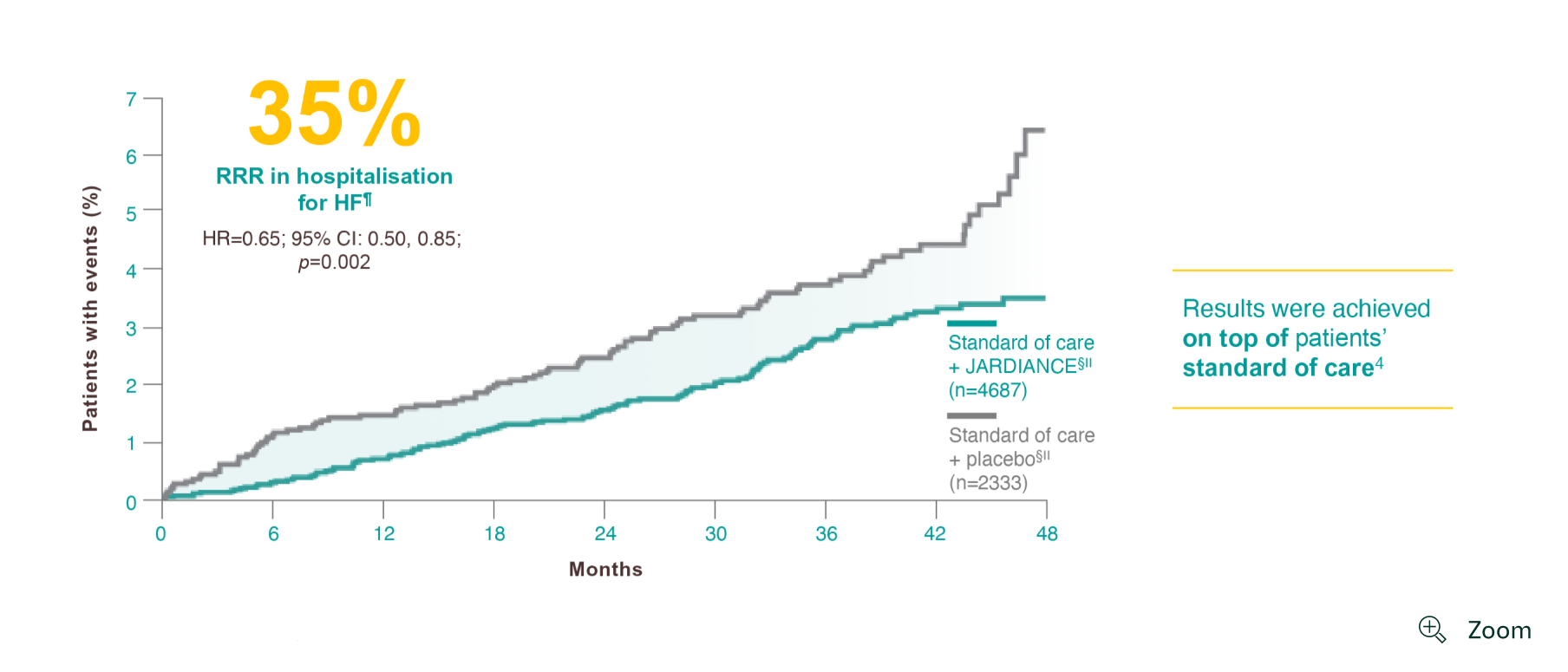
For your patients with eCVD and T2D*
Results from the EMPA-REG OUTCOME® trial.

For your patients with eCVD and T2D*
Results from the EMPA-REG OUTCOME® trial.
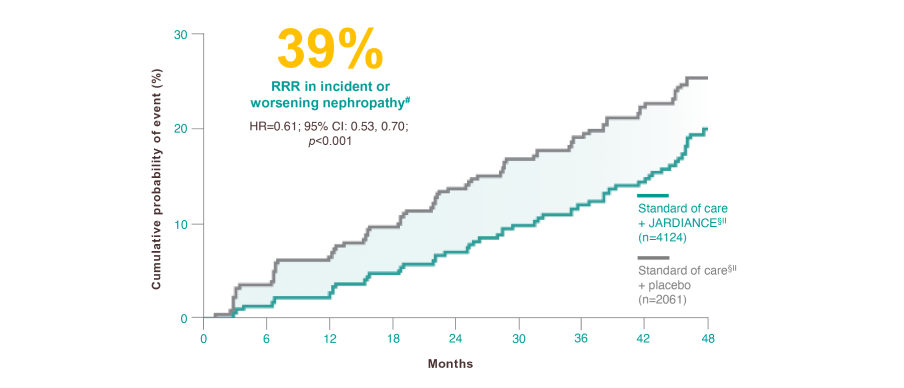
For your patients with eCVD and T2D*
Results from the EMPA REG OUTCOME ® trial.
EMPA-REG OUTCOME® focussed on patients with eCVD and T2D4
-
*
Adult patients with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke.4
-
†
In addition to reducing the risk of CV death when added to the standard of care, JARDIANCE also lowered HbA1c. In addition, JARDIANCE demonstrated reduction in weight and blood pressure. JARDIANCE is not indicated for weight loss or reduction of blood pressure.4
-
‡
CV death was part of the composite primary endpoint, 3-point MACE, in the EMPA-REG OUTCOME® trial (HR=0.86; 95% CI: 0.74, 0.99; p<0.001 for noninferiority; p=0.04 for superiority) and 38% RRR in CV death was achieved in the overall EMPA-REG OUTCOME® population for the duration of the trial (HR=0.62; 95% CI: 0.49, 0.77; p<0.001). There were no significant differences between the placebo and JARDIANCE groups of nonfatal MI (HR=0.87; 95% CI: 0.70, 1.09; p=0.22) or nonfatal stroke (HR=1.24; 95% CI: 0.92, 1.67; p=0.16).4
-
§
Pooled data from 10-mg and 25-mg doses of JARDIANCE; both doses showed a comparable reduction in the risk of CV death.4
-
II
Standard of care included CV medications and glucose-lowering agents given at the discretion of healthcare providers and according to recommendations of local guidelines.4
-
¶
Hospitalisation for HF was a secondary CV outcome in the EMPA-REG OUTCOME® trial (HR=0.65; 95% CI: 0.50, 0.85).4
-
#
Incident or worsening nephropathy is defined as progression to macroalbuminuria, doubling of serum creatinine, eGFR of ≤45 mL/min/1.73 m2; initiation of renal replacement therapy; death from renal disease. Incident or worsening nephropathy was a prespecified component of the secondary microvascular outcome in the EMPA-REG OUTCOME® trial.6
-
ACEi=angiotensin-converting enzyme inhibitor; ARR=absolute risk reduction; BMI=body mass index; CAD=coronary artery disease; CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; CVD=cardiovascular disease; eCVD=established cardiovascular disease; eGFR=estimated glomerular filtration rate; HbA1c=haemoglobin A1c; HF=heart failure; HR=hazard ratio; MACE=major adverse cardiovascular events; MI=myocardial infarction; NNT=number needed to treat; PAD=peripheral artery disease; PCP=primary care physician; RRR=relative risk reduction; SE=standard error of the mean; SGLT2i=sodium-glucose cotransporter-2 inhibitor; T2D=type 2 diabetes; uACR=urine albumin-to-creatinine ratio.
-
Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms. Diabetes Care. 2008;33(2):442-449.
-
Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, Fu SB. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21(74):1-15.
-
Cardiovascular disease. American Diabetes Association. Accessed May 1, 2024. https://diabetes.org/about-diabetes/complications/ cardiovascular-disease
-
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix.)
-
Verma S, Leiter LA, Sharma A, et al. How early after treatment initiation are the CV benefits of empagliflozin apparent? A post hoc analysis of EMPA-REG OUTCOME. Diabetes. 2020;69(suppl 1):28-OR.
-
Wanner Ch, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(18):1801-1802.
JARDIANCE is an easy choice to protect your patients by reducing CV and renal risk1
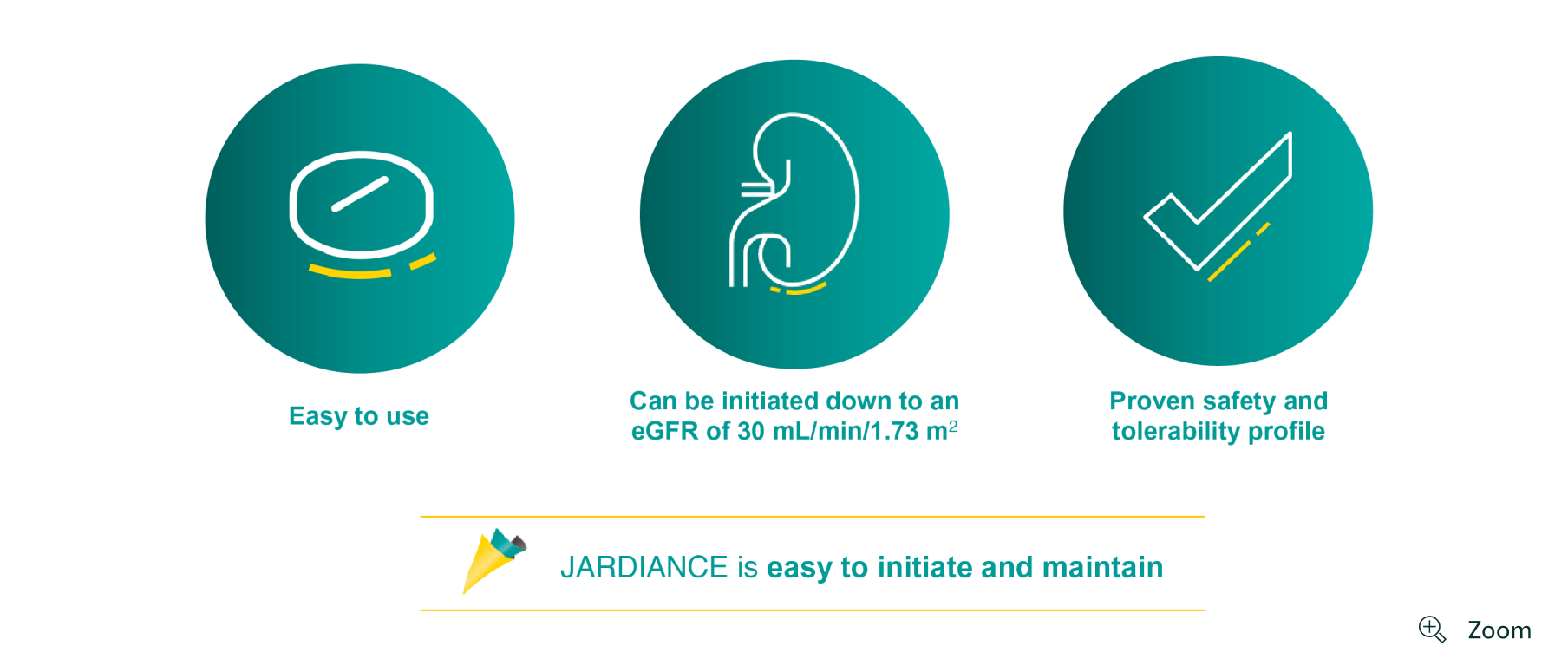
JARDIANCE can be initiated down to an eGFR of 301
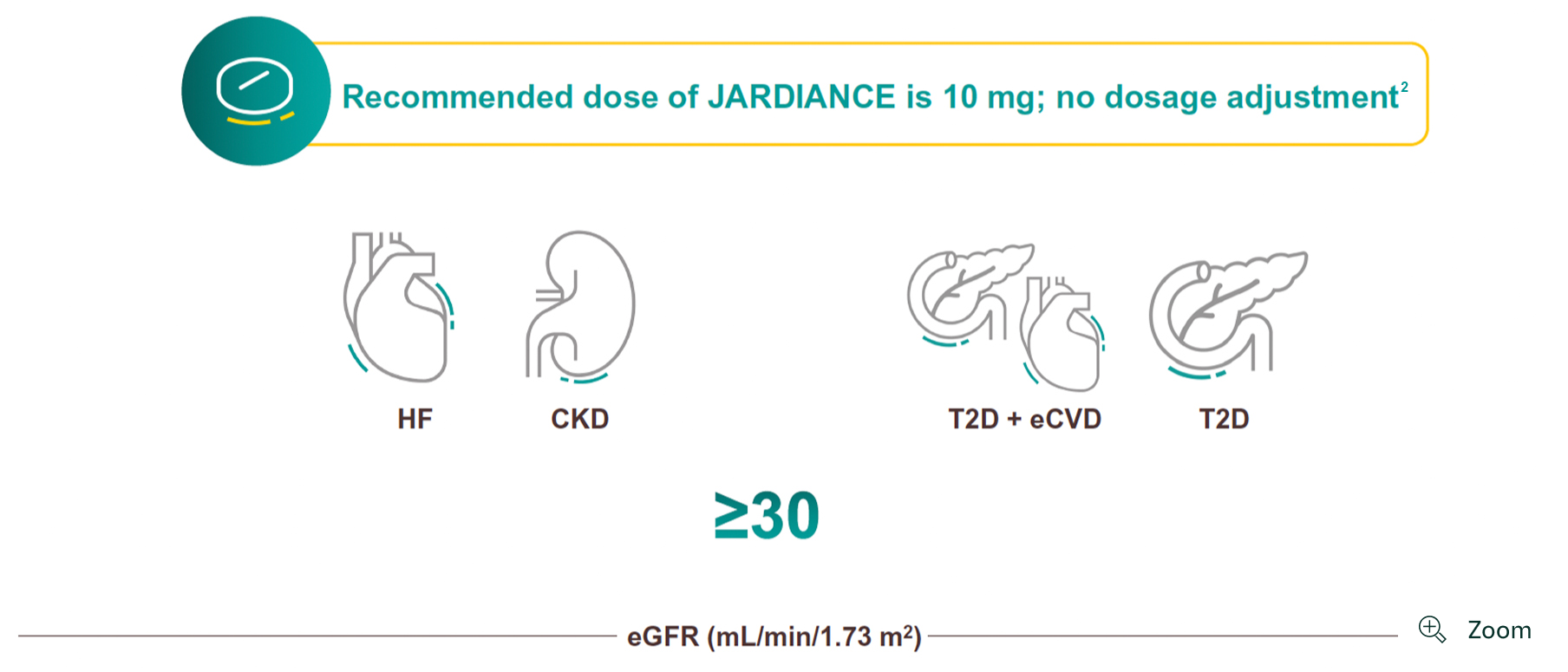
For full initiation and dosing details for patients with T2D with and without CVD, please refer to the Indian Prescribing Information.
JARDIANCE has a proven safety and tolerability profile across multiple trials†1
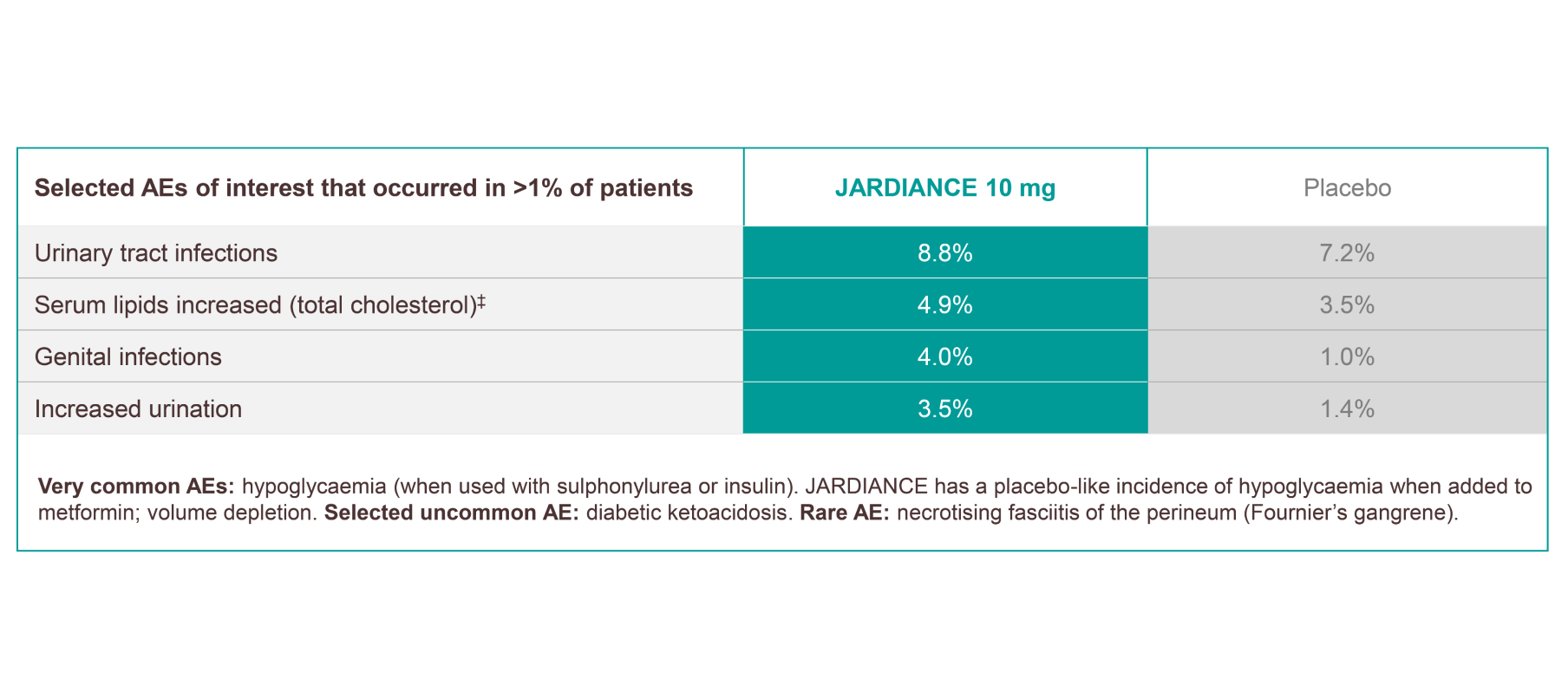
Frequencies are defined as very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100), rare (≥1/10,000 to <1/1000), and very rare (<1/10,000).
Contraindications and precautions
- JARDIANCE should not be used for the treatment of patients with:
– Type 1 diabetes
– Severe hepatic impairment
– Hypersensitivity to the active substance or to any of the excipients - In patients 75 years and older, an increased risk of volume depletion should be taken into account
- Due to limited experience, it is not recommended to initiate treatment with empagliflozin in patients with an eGFR <30 mL/min/1.73 m2
-
*
When JARDIANCE is used in combination with a sulphonylurea or with insulin, a lower dose of the sulphonylurea or insulin may be considered to reduce the risk of hypoglycaemia.1
-
†
Please see the Indian Prescribing Information for the full list of AEs and full list of special warnings and precautions for use.
-
‡
Mean percentage increase.
-
AE=adverse event; CKD=chronic kidney disease; CV=cardiovascular; CVD=cardiovascular disease; eCVD=established cardiovascular disease; eGFR=estimated glomerular filtration rate; HF=heart failure; SmPC=Summary of Product Characteristics; T2D=type 2 diabetes.
-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
-
Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568-574.
