Why CRM?
For patients with CV disease and type 2 diabetes*
JARDIANCE® helps you DO MORE than reduce HbA1c1,2
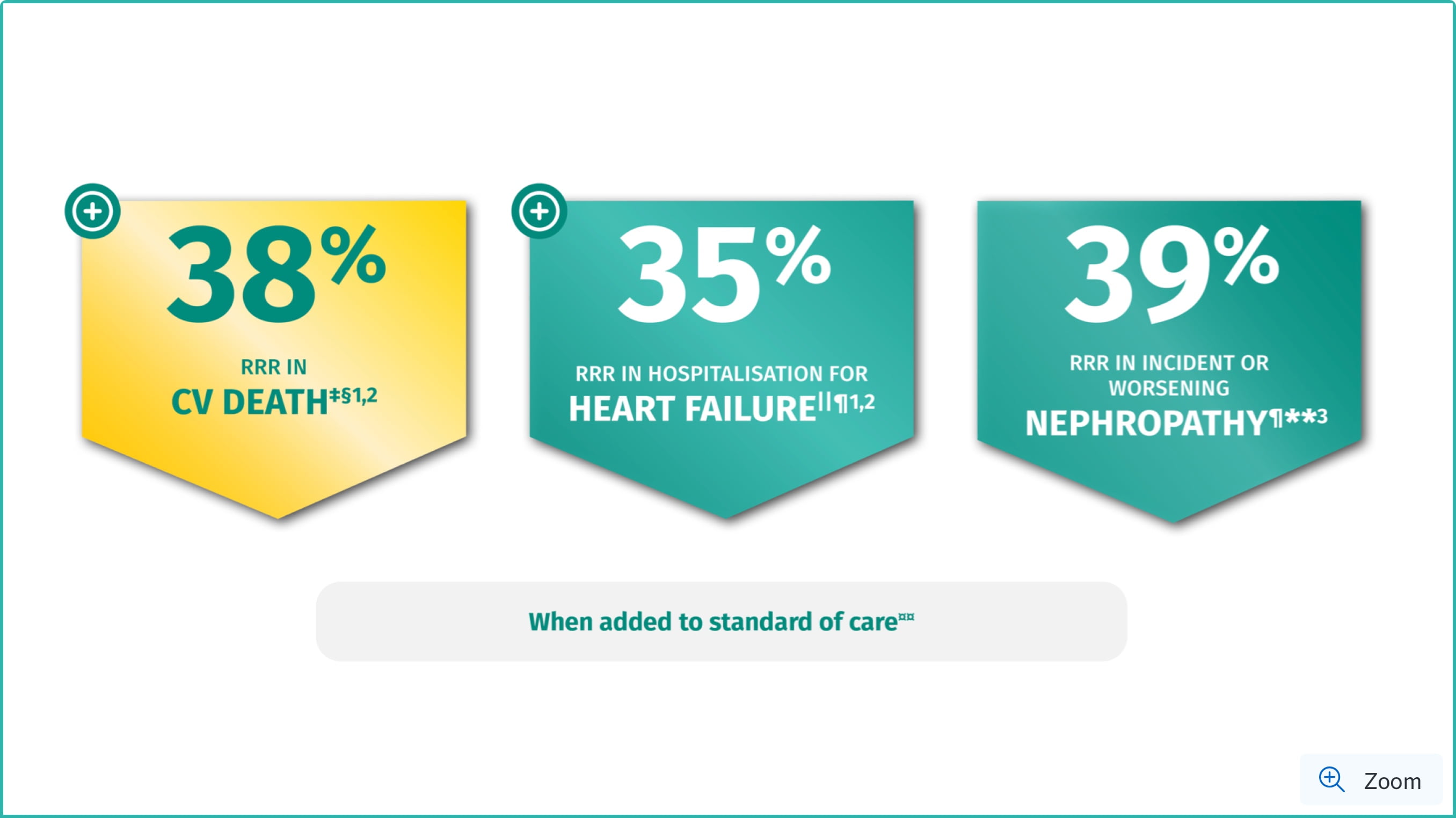
RRR in CV death: HR=0.62; 95% CI: 0.49, 0.77; p<0.001.1,2
RRR in hospitalisation for heart failure: HR=0.65; 95% CI: 0.50, 0.85; p=0.002.1,2
RRR in incident or worsening nephropathy: HR=0.61; 95% CI: 0.53, 0.70; p<0.001.3
-
JARDIANCE® prescribing information, version 20-Jan-2023. Boehringer Ingelheim India Pvt Ltd.
-
Data on file. Boehringer Ingelheim Pharmaceuticals, Inc.
-
Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650-1659.
-
*Adult patients with insufficiency controlled type 2 diabetes and CAD, PAD, or a history of MI or stroke.1,2
-
‡The 38% RRR in CV death was achieved in the overall EMPA-REG OUTCOME® population for the duration of the trial (HR=0.62; 95% CI: 0.49, 0.77; p<0.001).2
-
‡Pooled data from 10-and 25-mg doses of JARDIANCE®; both doses showed a comparable reduction in the risk of CV death.1,2
-
‡Hospitalization for heart failure was a secondary CV outcome in the EMPA-REG OUTCOME® trial (HR=0.65; 95% Cl: 0.50, 0.85; p=0.002).2
-
‡Pooled data from 10 mg and 25 mg doses of JARDIANCE®; both doses showed a comparable reduction in the risk of hospitalization for heart failure and incident or worsening nephropathy.1,3
-
**Incident or worsening nephropathy is defined as progression to macroalbuminuria, doubling of serum creatinine, eGFR of ≤45 mL/min/1.73 m2; initiation of renal replacement therapy; death from renal disease. Incident or worsening nephropathy was a prespecified component of the secondary microvascular CV outcome in the EMPA-REG OUTCOME® trial (HR=0.61; 95% CI: 0.53, 0.70; p<0.001) for JARDIANCE® (12.7%) versus placebo (18.8%).3
-
‡Standard of care included CV medications and glucose-lowering agents given at the discretion of physicians.1,2
-
ACEi=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; CAD=coronary artery disease; CI=confidence interval; CV=cardiovascular; eGFR=estimated glomerular filtration rate; HR=hazard ratio; MI=myocardial infarction; PAD=peripheral artery disease; RRR=relative risk reduction.
For patients with CV disease and type 2 diabetes*
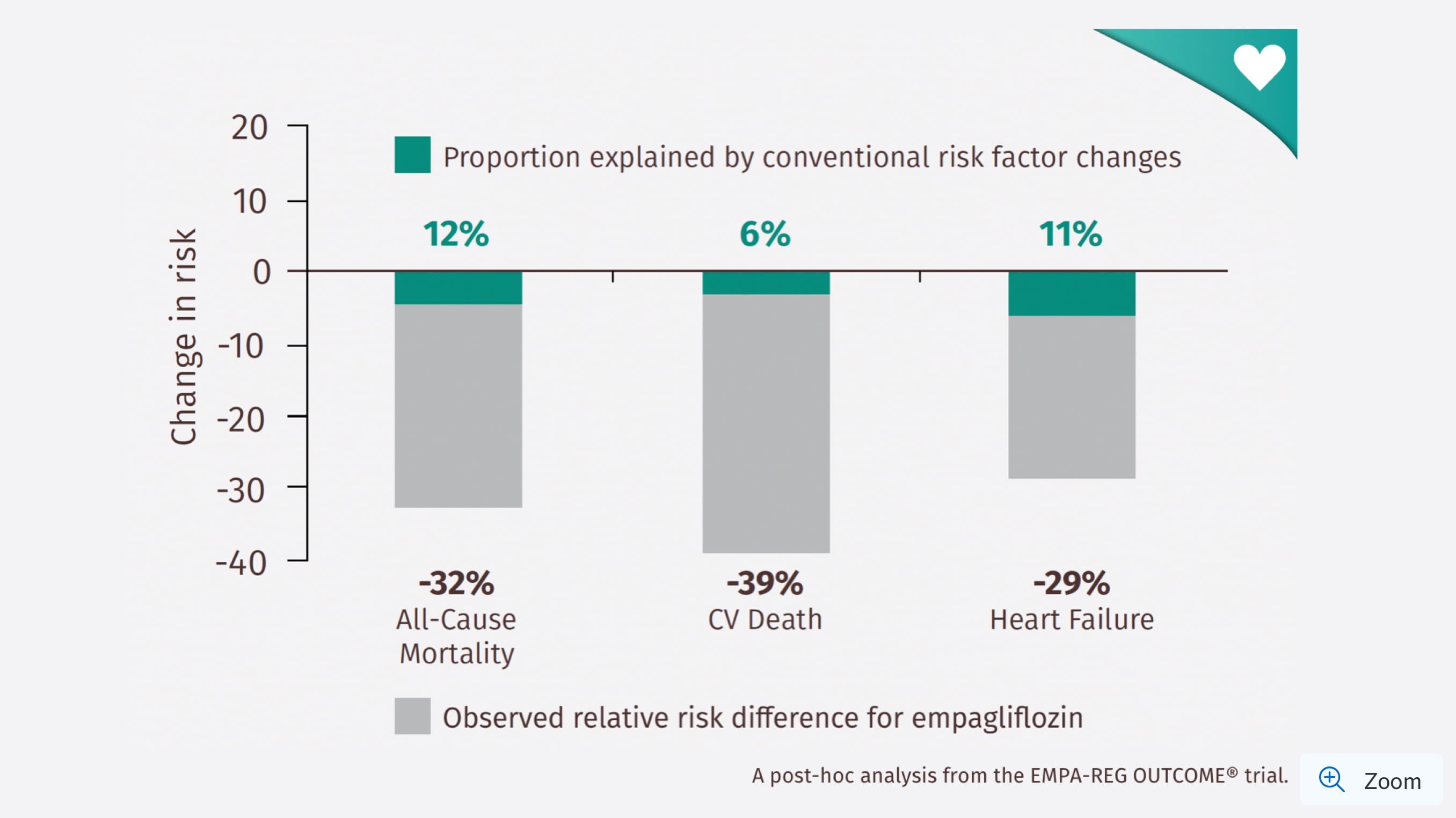
CV benefits observed with JARDIANCE® are primarily driven by mechanisms beyond conventional CV risk factor¤ reductions1
Proportion of observed risk reductions attributable to changes in conventional risk factors‡§1
-
Coleman RL, Gray AM, Broedl UC, et al. Can the cardiovascular risk reductions observed with empagliflozin in the EMPA-REG OUTCOME trial be explained by concomitant changes seen in conventional cardiovascular risk levels? Diabetes Obes Metab. 2020;22(7):1151-1156.
-
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin,
cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
(EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix.)
-
*Adult patients with insufficiently controlled type 2 diabetes and CAD, PAD, or a history of MI or stroke.2
-
†Conventional risk factors included HbA1c, cholesterol, systolic BP, and presence of comorbidities.
-
‡Post hoc analyses of 3-year EMPA-REG OUTCOME® CV event rates using a T2D-specific clinical outcomes simulation model applied to annual patient-level data.
-
§Percentage values shown are observed risk reductions for JARDIANCE® for each outcome (grey bars) and the proportion of these that is explained by the changes to conventional risk factors (green bars).
-
BP, blood pressure; CAD, coronary artery disease; CI, confidence interval; CV, cardiovascular; HbA1c, glycated haemoglobin; HR, hazard ratio; MI, myocardial infarction; P, prevalence; PAD, peripheral artery disease; T2D, type 2 diabetes.
For patients with CV disease and type 2 diabetes*
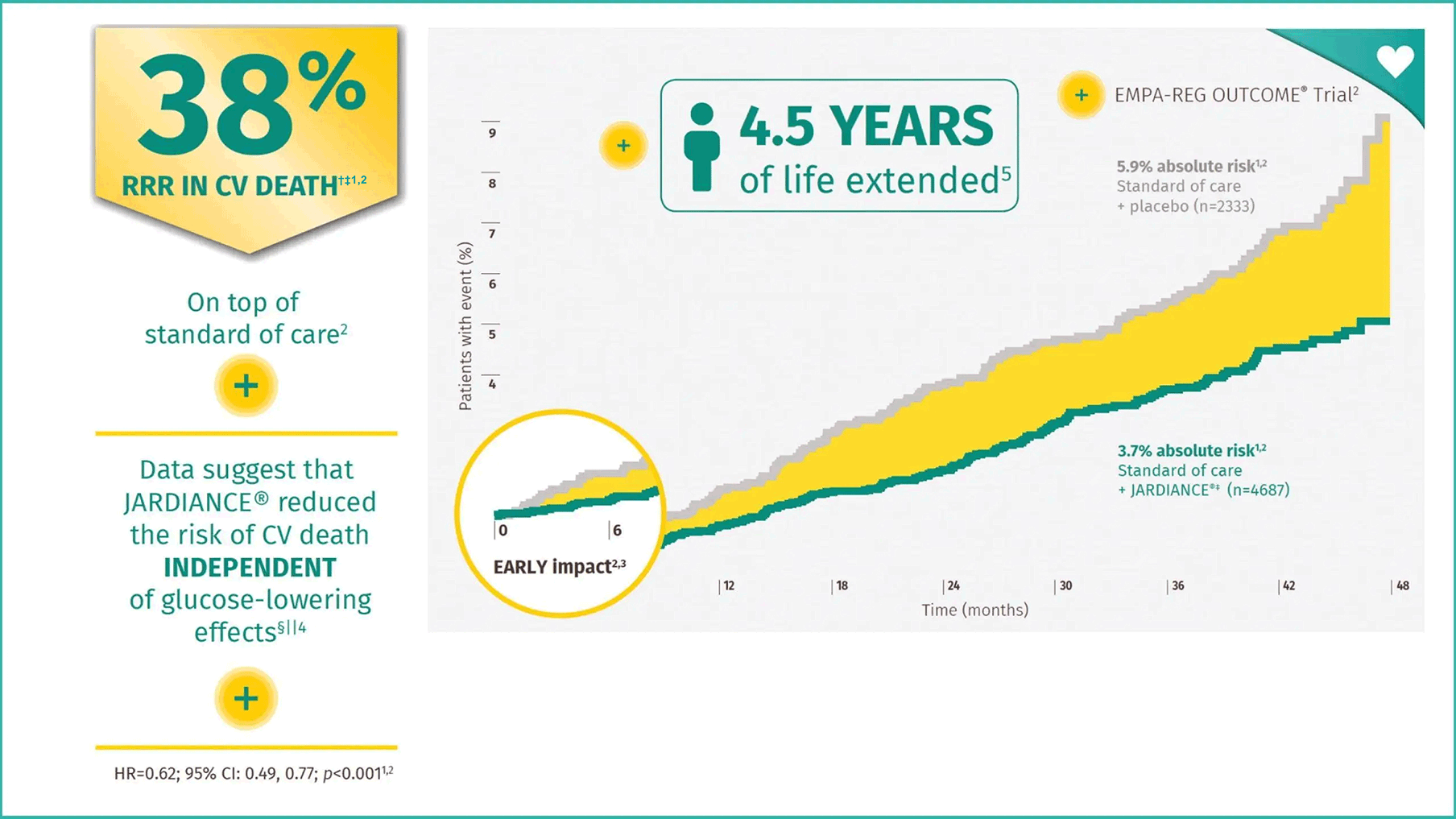
JARDIANCE® demonstrated reduction in the risk of CV death- early and sustained1,2
For patients with type 2 diabetes and CV disease*
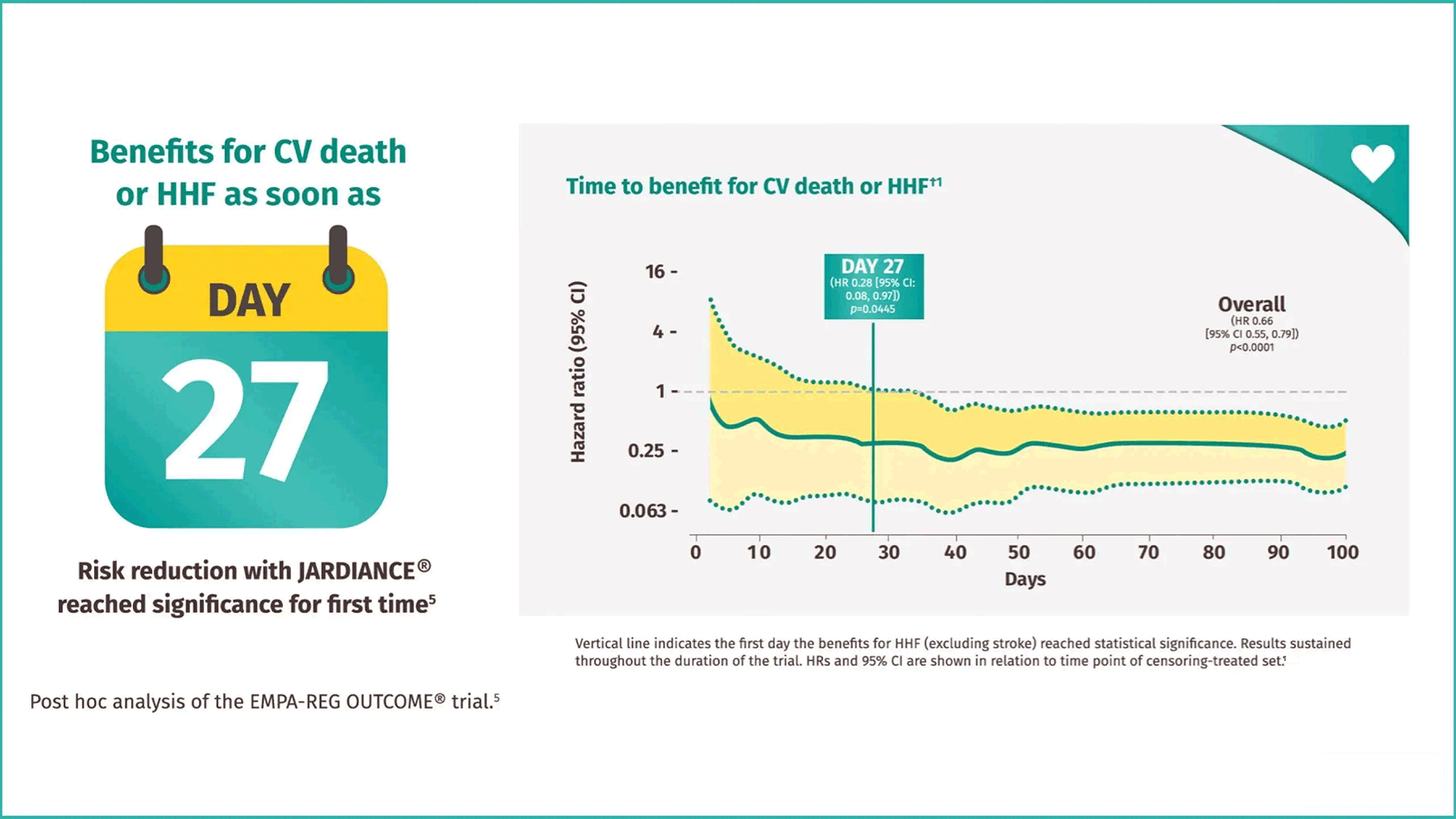
JARDIANCE® reduced the risk of CV death or HHF in as early as
1 month5
For your patients with type 2 diabetes and CV disease*
JARDIANCE® reduced the risk of CV death in as early as
2 months5
In EMPA-REG OUTCOME®, JARDIANCE® reduced the relative risk of CV death by 38%1,2

-
JARDIANCE® prescribing information, version 20-Jan-2023. Boehringer Ingelheim India Pvt Ltd.
-
Zinman B, Wanner C, Lachin J, et al; EMPA-REG OUTCOME® Investigators. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix for certain baseline characteristics).
-
Fitchett D, Inzucchi SE, Lachin JM, et al; EMPA-REG OUTCOME® Investigators. Cardiovascular mortality reduction with empagliflozin in patients with type 2 diabetes and cardiovascular disease. J Am Coll Cardiol. 2018;71(3):364-367.
-
Inzucchi SE, Kosiborod M, Fitchett D, et al. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation. 2018;138:1904-1907.
-
Claggett B, Lachin JM, Hantel S, et al. Long-term benefit of empagliflozin on life expectancy in patients with type 2 diabetes mellitus and established cardiovascular disease: Survival estimates from the EMPA-REG OUTCOME trial. Circulation. 2018;138:1599-1601.
-
*Adult patients with insufficiently controlled type 2 diabetes and CAD, PAD, or a history of MI or stroke.1,2
-
†The 38% RRR in CV death was achieved in the overall EMPA-REG OUTCOME® population for the duration of the trial (HR=0.62; 95% CI: 0.49, 0.77; p<0.001).1,2
-
‡Pooled data from 10 mg and 25 mg doses of JARDIANCE®; both doses showed a comparable reduction in the risk of CV death.1,2
-
§A post-hoc analysis of data from the EMPA-REG OUTCOME® trial by baseline HbA1c subgroups. EMPA-REG OUTCOME® was not powered to show differences between subgroups.2,5
-
||Hazard ratios are related to CV death: HbA1c <7% HR=0.30 (95% CI: 0.12, 0.80); HbA1c 7% to <8% HR=0.59 (95% CI: 0.42, 0.83); HbA1c 8% to <9% HR=0.67 (95% CI: 0.45, 0.99); HbA1c ≥9% HR=0.76 (95% CI: 0.44, 1.31); p=0.41 for interaction.5
-
¶Standard of care included CV medications and glucose-lowering agents given at the discretion of physicians.
-
ACEi=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; CAD=coronary artery disease; CI=confidence interval; CV=cardiovascular; HbA1c=hemoglobin A1c; HR=hazard ratio; MI=myocardial infarction; PAD=peripheral artery disease; RRR=relative risk reduction.

EMPAGLIFLOZIN REAL-WORLD EFFECTIVENESS
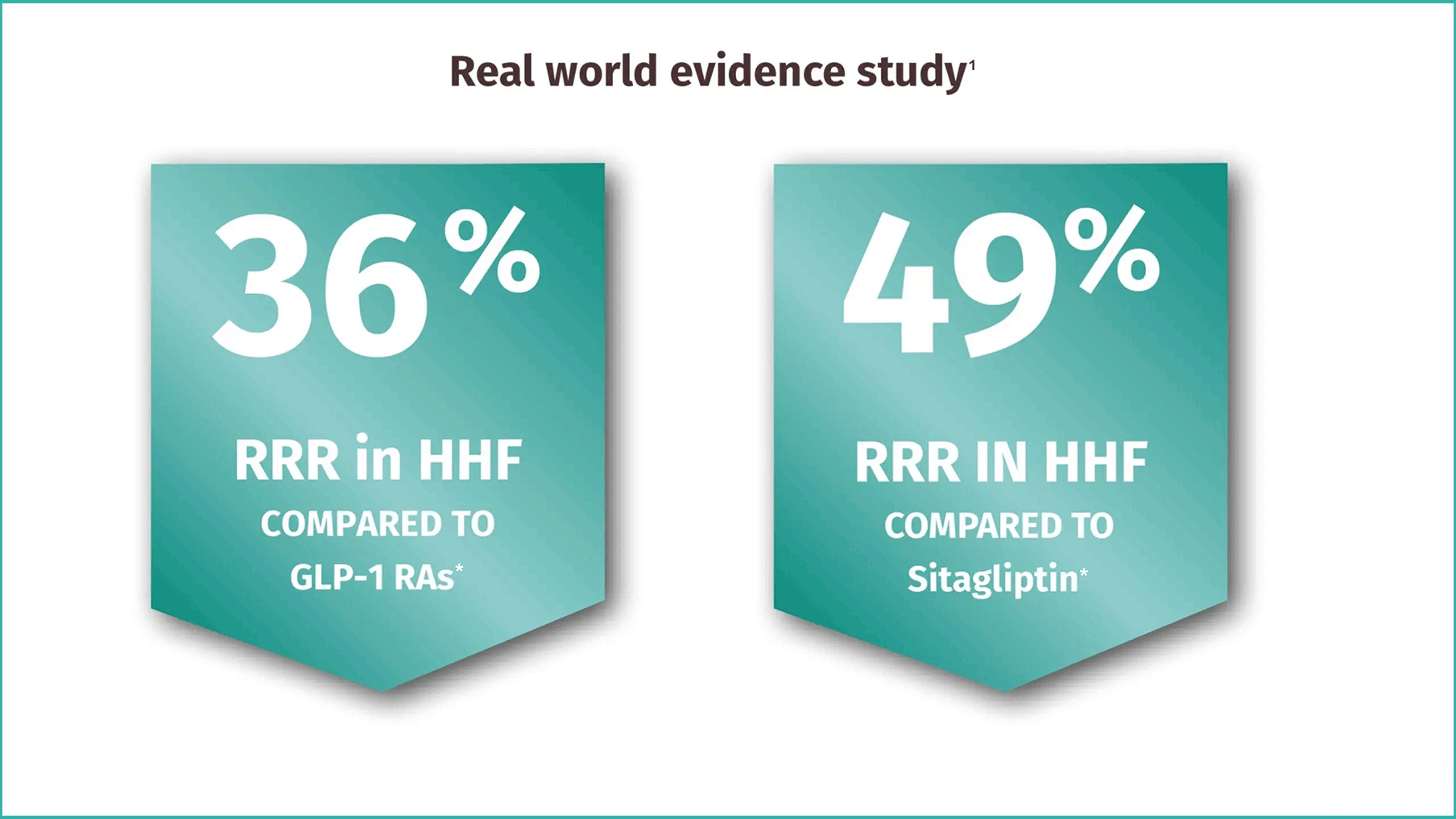
-
Approx. 33,000 patients with type 2 diabetes
-
75% without CV disease
-
EMPRISE is a five year study program on the effectiveness, safety and healthcare utilization of empagliflozin in routine care across a spectrum of cardiovascular baseline risk using real-world data from three U.S. claims datasets
-
Patorno E, Najafzadeh M, Pawar A, et al. The EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study programme: Design and exposure accrual for an evaluation of empagliflozin in routine clinical care. Endocrinol Diabetes Metab. 2019;3(1):e00103.
-
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix.)
-
*In addition to reducing the risk of CV death when added to the standard of care, JARDIANCE® also lowered glucose levels. In addition, JARDIANCE® demonstrated reduction in weight and blood pressure. JARDIANCE® is not indicated for weight loss or reduction in blood pressure.2
-
CI, confidence interval; CV, cardiovascular; GLP-1 RA; glucagon-like peptide-1 receptor agonists; HHF, heart failure hospitalization; HR, hazard ratio; RRR, relative risk reduction; US, United States
PC-IN-103695
