Why CRM?
For your patients with T2D and eCVD*
JARDIANCE® helped reduce the risk of incident or worsening nephropathy†1
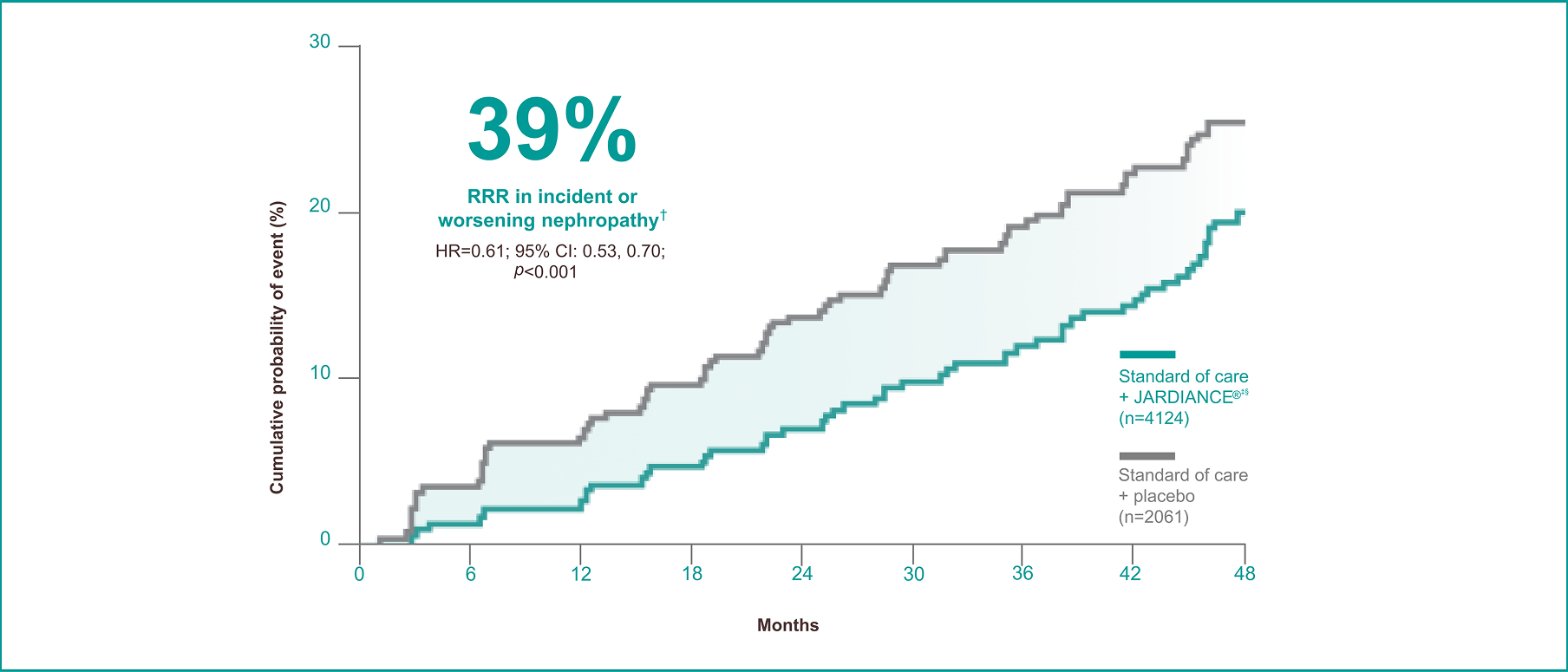
Results from the EMPA-REG OUTCOME® trial.
For your patients with T2D and eCVD*
JARDIANCE® slowed the decline in kidney function†1
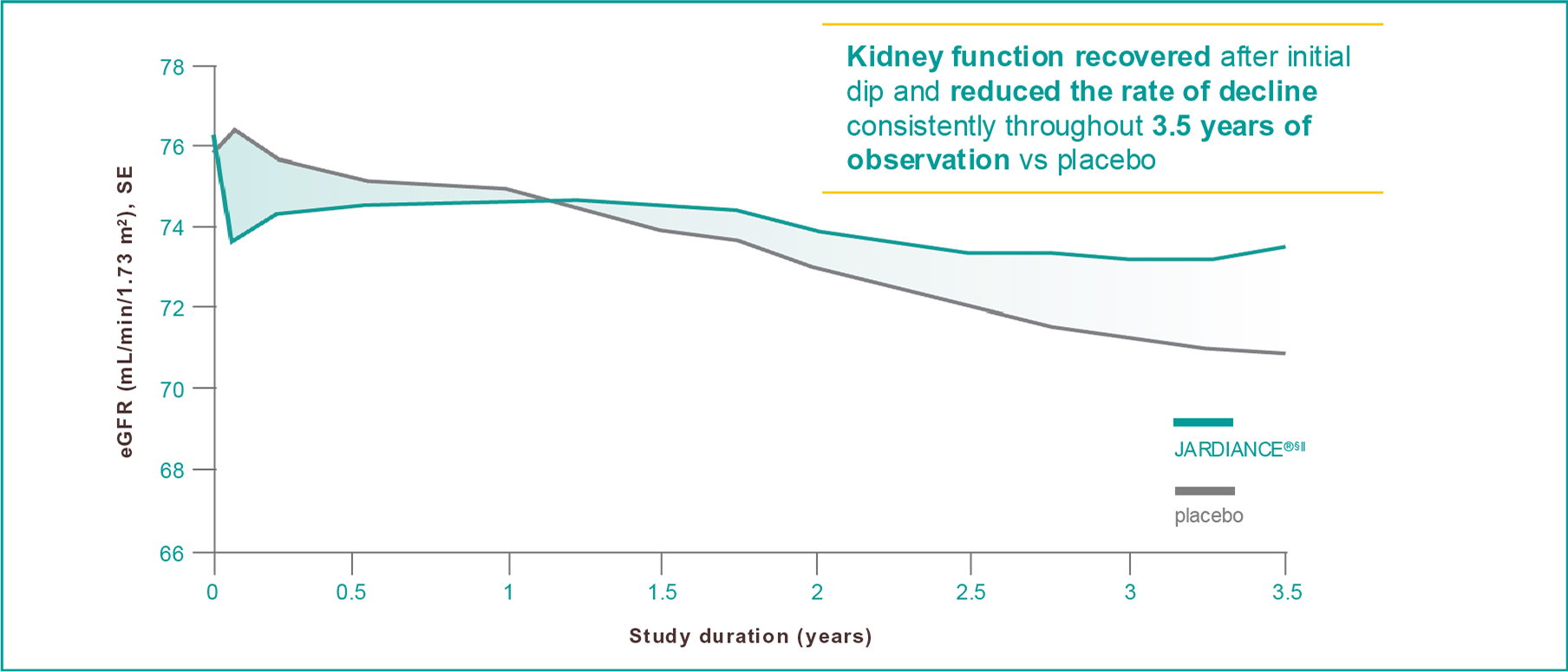
Results from the EMPA-REG OUTCOME® trial.
In the treatment of patients with CKD with or without T2D||
JARDIANCE® reduced kidney disease progression or risk of CV death||2
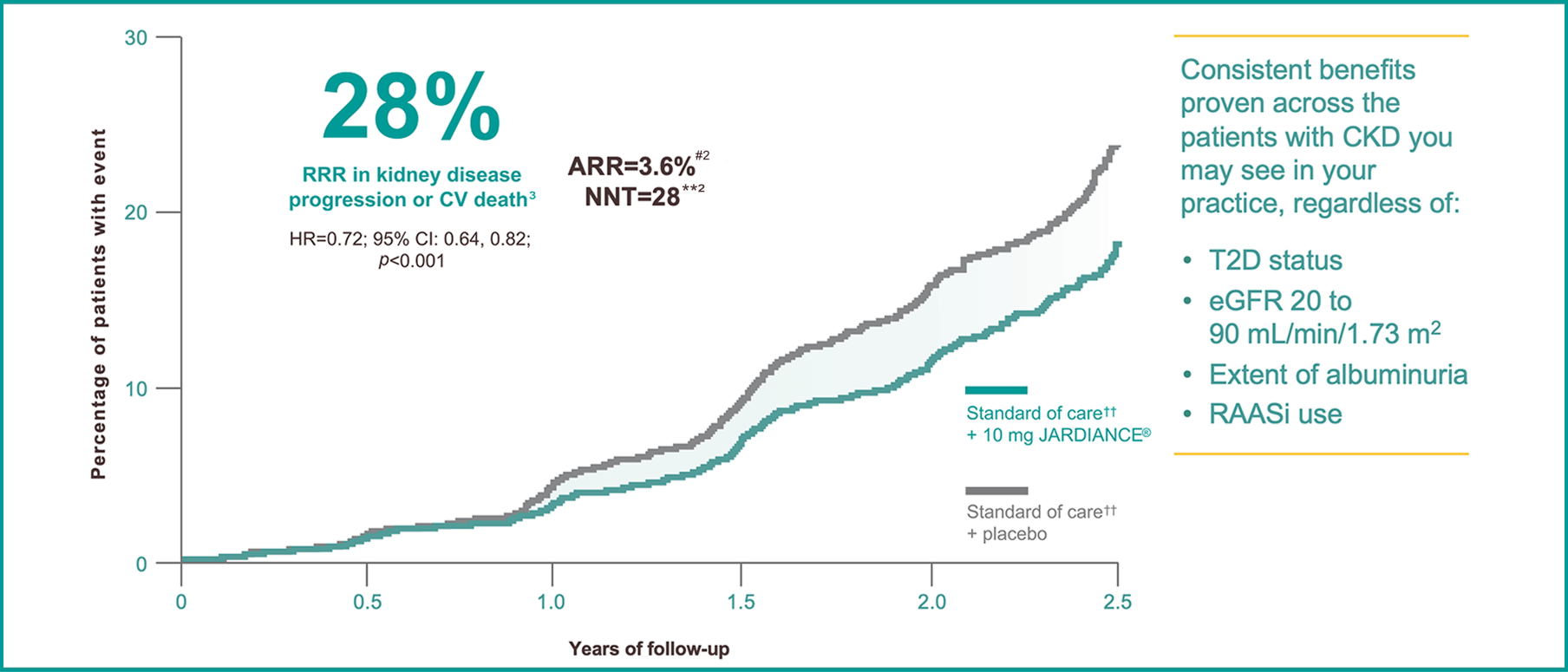
Results from the EMPA-KIDNEY trial.
According to a calculation based on the hypothetical transformation of chronic eGFR slopes into time to kidney failure in an independent publication,
JARDIANCE® provides the greatest protection to kidney health and delay of dialysis when initiated early4
Extrapolated data from EMPA-KIDNEY shows that JARDIANCE® may slow onset of dialysis by >20 years, compared to placebo, with efficacy across a range of eGFR values.
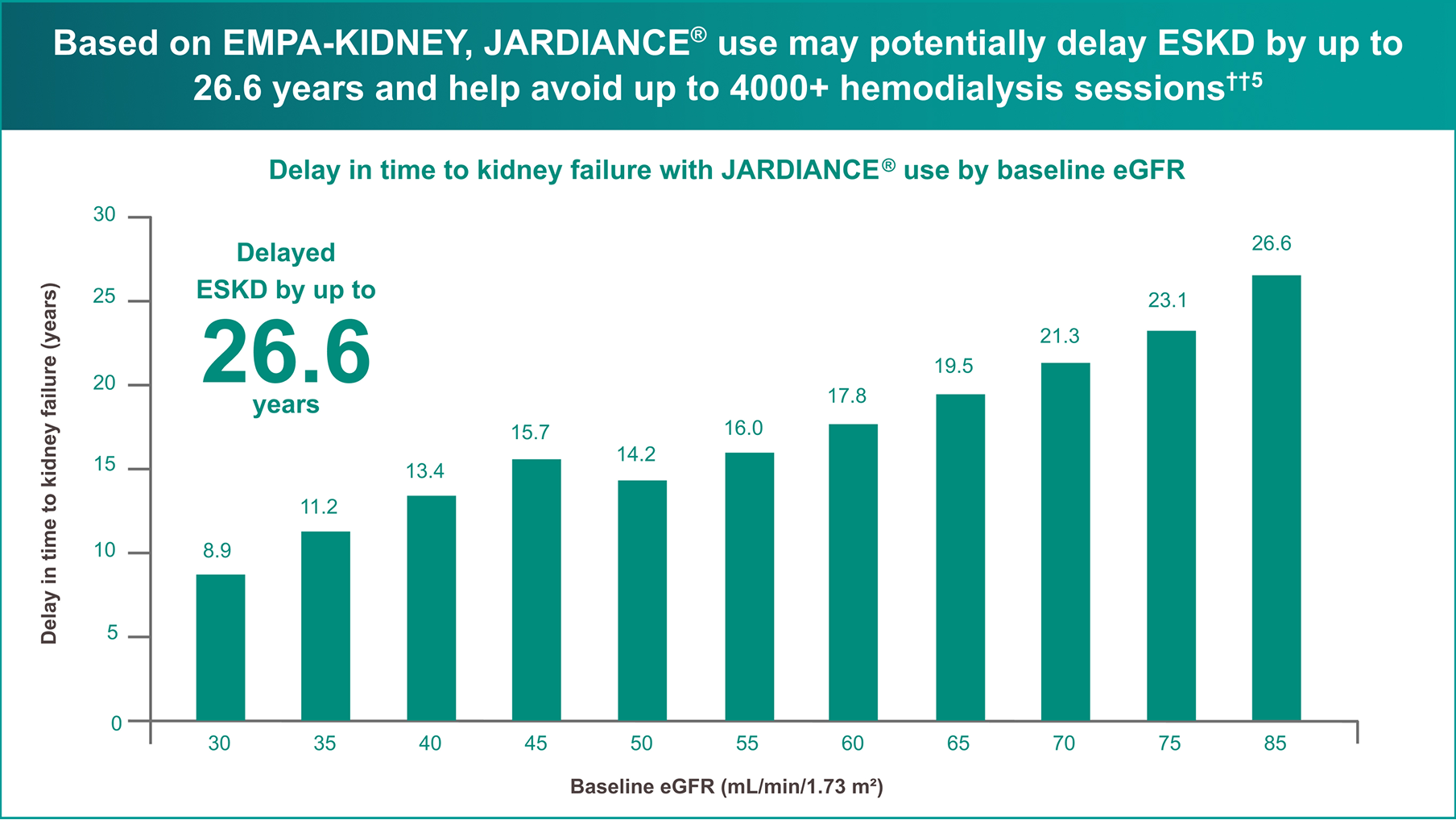
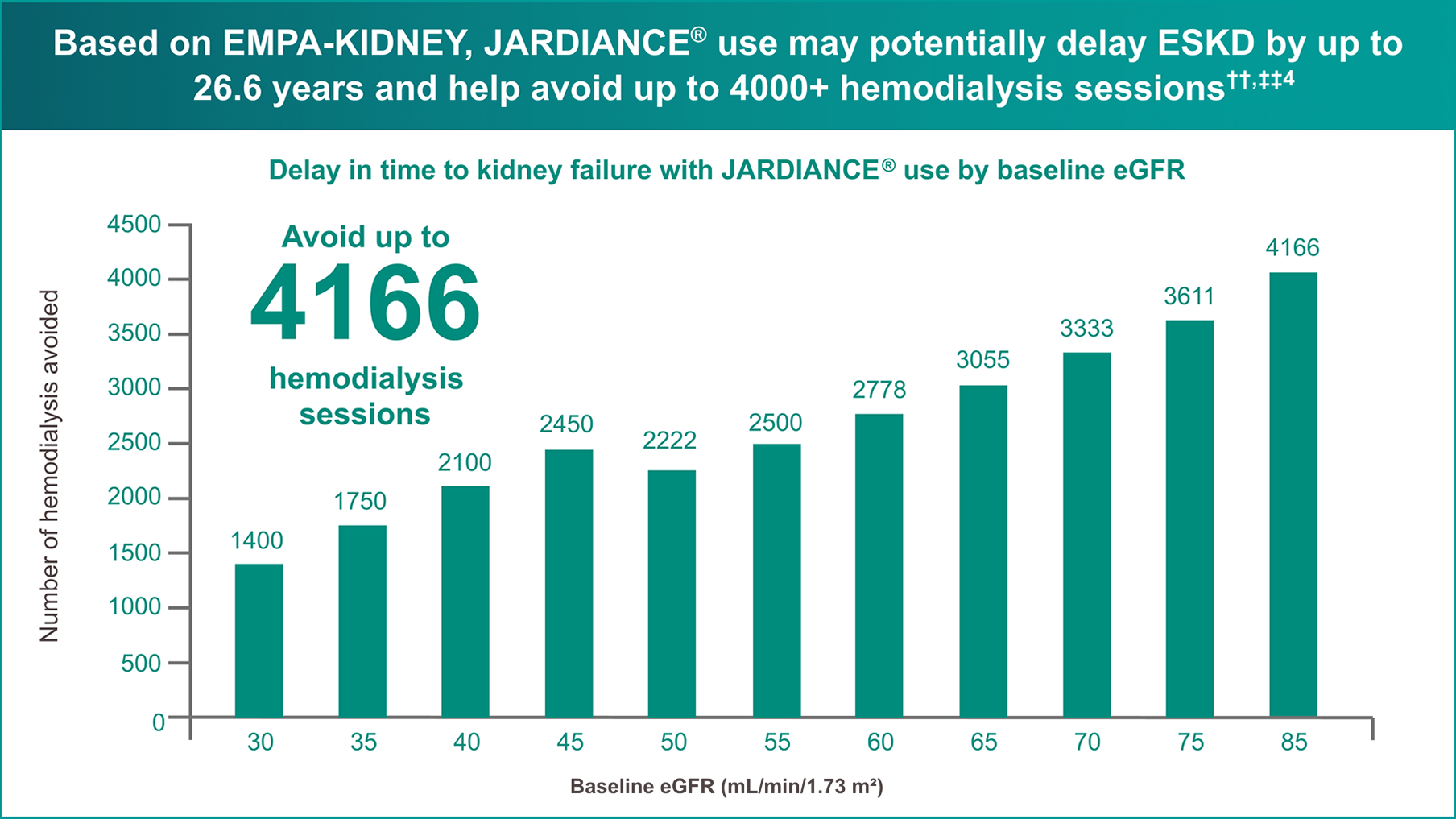

Patients with CKD can benefit in early (eGFR ≥60) and late stages of disease progression.
-
Wanner C, Inzucchi SE, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334.
-
Herrington WG, Staplin N, Wanner C, et al. EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. (EMPA-KIDNEY results and the publication’s Supplementary Appendix.)
-
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix.)
-
Fernández-Fernandez B, Sarafidis P, Soler MJ, Ortiz A. EMPA-KIDNEY: expanding the range of kidney protection by SGLT2 inhibitors. Clin Kidney J. 2023;16(8):1187-1198.
-
*
Adult patients with insufficiently controlled T2D and CAD, PAD, or a history of MI or stroke.3
-
†
Incident or worsening nephropathy is defined as progression to macroalbuminuria, doubling of serum creatinine, eGFR of ≤45 mL/min/1.73 m2; initiation of renal replacement therapy; death from renal disease. Incident or worsening nephropathy was a prespecified component of the secondary microvascular outcome in the EMPA-REG OUTCOME® trial. The primary composite outcome in the EMPA-REG OUTCOME® trial was 3-point MACE.1,3
-
‡
Standard of care included CV medications and glucose-lowering agents given at the discretion of healthcare providers and according to recommendations of local guidelines.3
-
§
Pooled data from 10-mg and 25-mg doses of JARDIANCE®; both doses showed a comparable reduction in the risk of CV death.1
-
||
Adult patients with an eGFR ≥20, <45 mL/min/1.73 m2 or an eGFR ≥45, <90 mL/min/1.73 m2 with a uACR ≥200 mg/g.2
-
¶
In the EMPA-KIDNEY trial, a randomised, parallel-group, double-blind, placebo-controlled study of 6609 patients with CKD, the efficacy and safety of JARDIANCE® 10 mg (n=3304) were evaluated vs placebo (n=3305). The primary endpoint in the EMPA-KIDNEY trial was a composite of CV death or progression of kidney disease. Patients treated with JARDIANCE® experienced a 28% RRR in this endpoint (HR=0.72; 95% CI: 0.64, 0.82; p<0.001).2
-
#
ARR for the primary composite outcome of kidney disease progression or CV death is 3.6% per patient-year at risk. Figure adapted from Herrington et al.2
-
**
NNT: 28 (95% CI: 19, 53) per 2 years at risk.2
-
††
Estimated from each baseline eGFR value by applying the chronic eGFR slopes corresponding to participants on placebo vs JARDIANCE® within the prespecified eGFR subgroups (eGFR cutoff points to define subgroups).4
-
‡‡
Standard of care: All patients received a RAASi unless an investigator judged that a RAASi was not indicated or tolerated.2
-
ARR, absolute risk reduction; CI, confidence interval; CKD, chronic kidney disease; CRM, cardio renal and metabolic; CV, cardiovascular; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HR, hazard ratio; NNT, number needed to treat; P, probability; RAASi, renin‐angiotensin‐aldosterone system inhibitors; RRR, relative risk reduction; T2D, type 2 diabetes.
PC-IN-103695
