Why CRM?
JARDIANCE® is an easy choice to protect your patients by reducing CV and renal risk1
Systolic BP
JARDIANCE® has a proven safety and tolerability profile across multiple trials*1
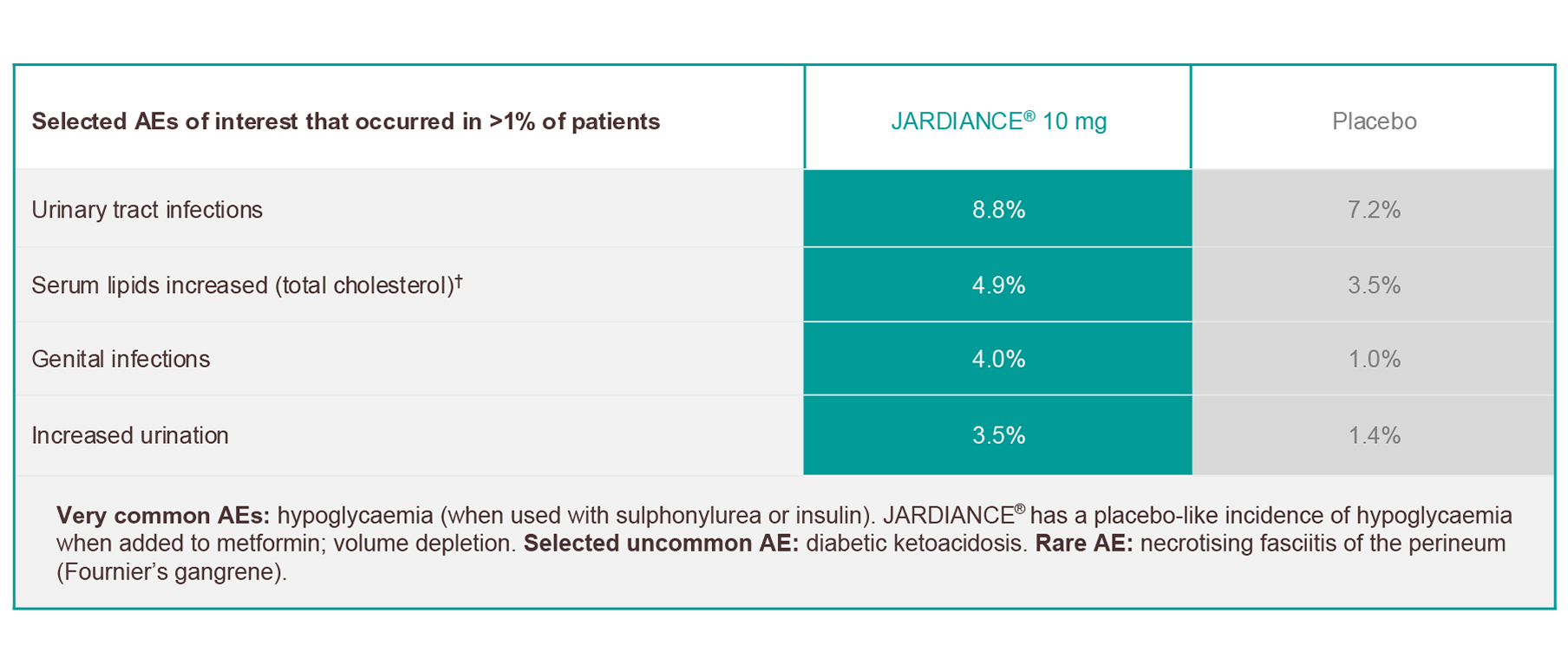
Frequencies are defined as: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1000 to <1/100); rare (≥1/10,000 to <1/1000); very rare (<1/10,000).
Contraindications and precautions
- JARDIANCE® should not be used for the treatment of patients with:
– Type 1 diabetes
– Severe hepatic impairment
– Hypersensitivity to the active substance or to any of the excipients - In patients 75 years and older, an increased risk of volume depletion should be taken into account
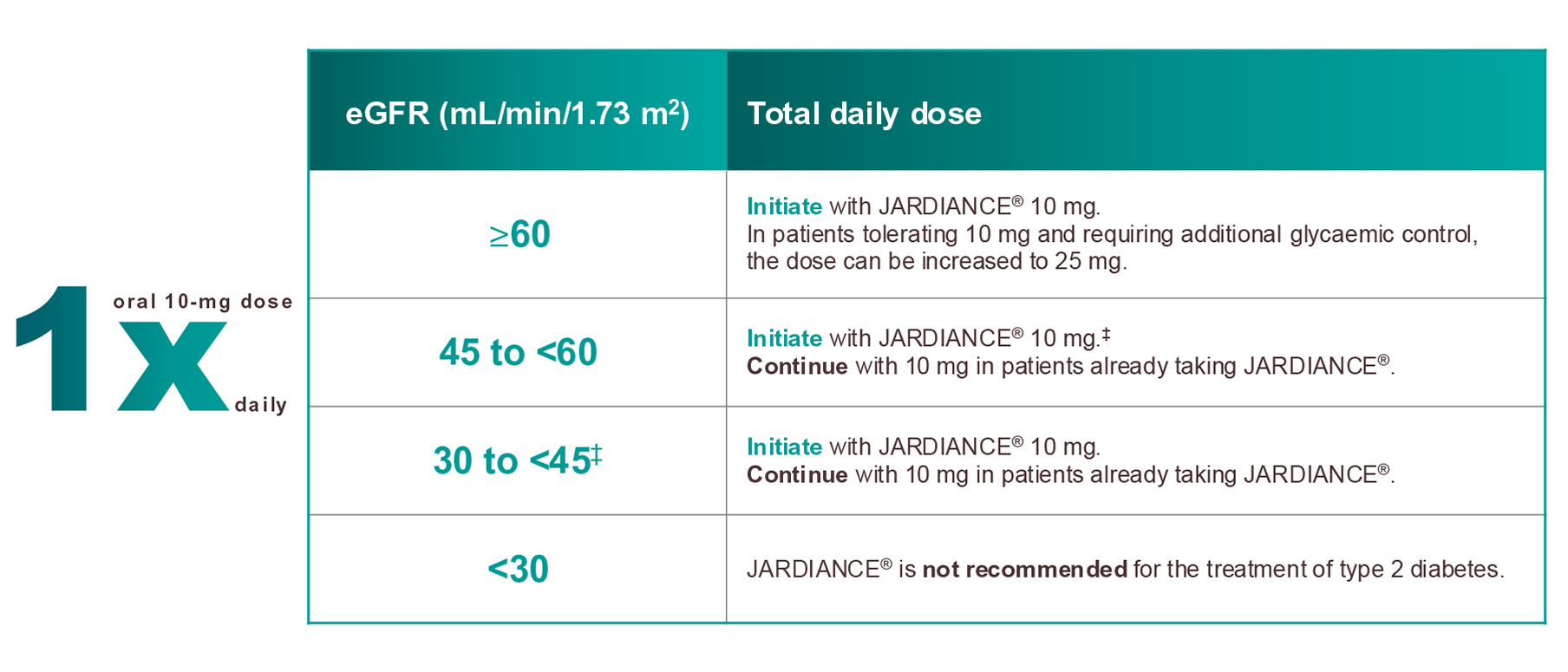
- Due to limited experience, it is not recommended to initiate treatment with JARDIANCE® in patients with an eGFR <20 mL/min/1.73 m2

For your patients with T2D
For patients with CV disease and type 2 diabetes
JARDIANCE® helps you DO MORE than reduce HbA1c2,3
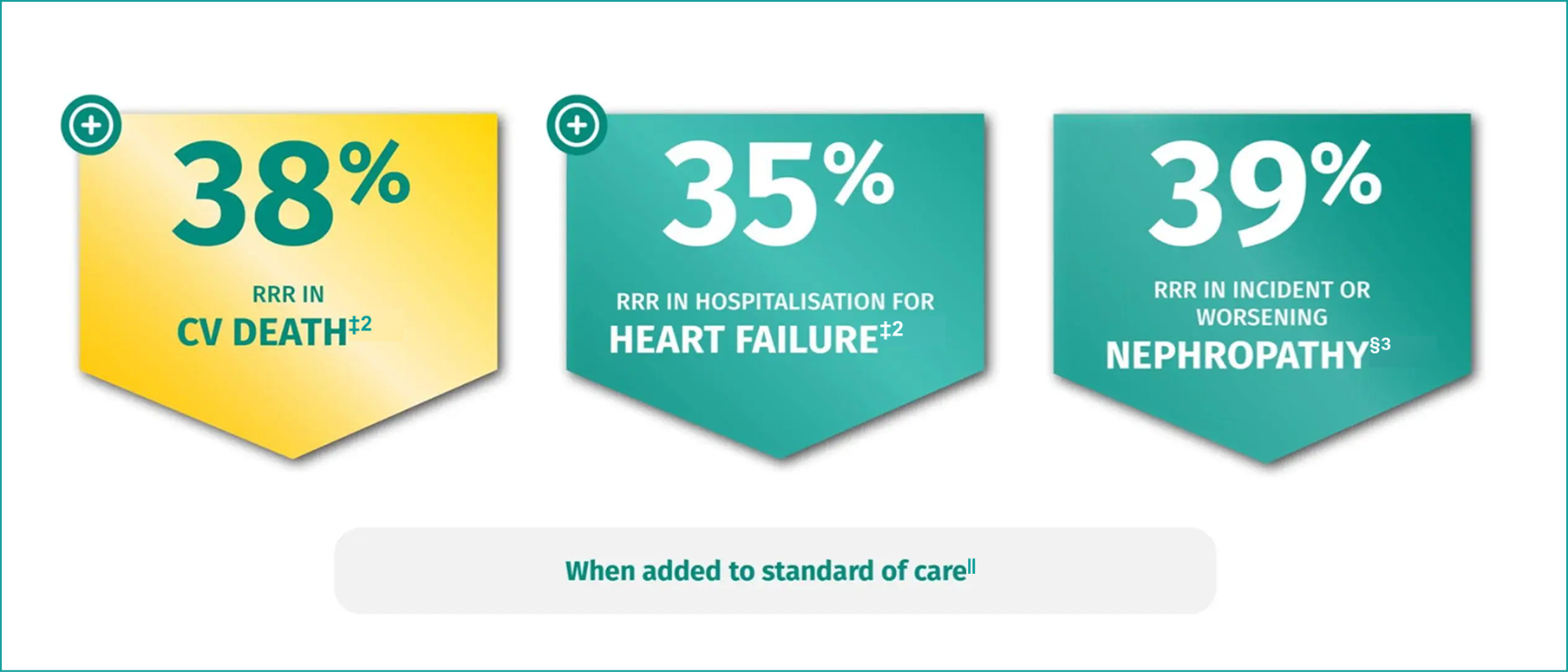
RRR in CV death: HR=0.62; 95% CI: 0.49, 0.77; p<0.001.2
RRR in hospitalisation for heart failure: HR=0.65; 95% CI: 0.50, 0.85; p=0.002.2
RRR in incident or worsening nephropathy: HR=0.61; 95% CI: 0.53, 0.70; p<0.001.3
-
API dated 19th Jul 2024 (Based on JARDIANCE® approved prescribing information dated 18th Jul 2024).
-
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. (EMPA-REG OUTCOME® results and the publication’s Supplementary Appendix.)
-
Wanner C, Inzucchi SE, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334.
-
*
Please see the SmPC for the full list of AEs and full list of special warnings and precautions for use, as well as dosing details.
-
†
Patients with T2D and eCVD.
-
‡
CV death was part of the composite primary endpoint, 3-point MACE, in the EMPA-REG OUTCOME® trial (HR=0.86; 95% CI: 0.74, 0.99; p<0.001 for noninferiority; p=0.04 for superiority) and 38% RRR in CV death was achieved in the overall EMPA-REG OUTCOME® population for the duration of the trial (HR=0.62; 95% CI: 0.49, 0.77; p<0.001). There were no significant differences between the placebo and JARDIANCE® groups of nonfatal MI (HR=0.87; 95% CI: 0.70, 1.09; p=0.22) or nonfatal stroke (HR=1.24; 95% CI: 0.92, 1.67; p=0.16).2
-
§
Incident or worsening nephropathy is defined as progression to macroalbuminuria, doubling of serum creatinine, eGFR of ≤45 mL/min/1.73 m2; initiation of renal replacement therapy; death from renal disease. Incident or worsening nephropathy was a prespecified component of the secondary microvascular outcome in the EMPA-REG OUTCOME® trial. The primary composite outcome in the EMPA-REG OUTCOME® trial was 3-point MACE.3
-
||
Standard of care included CV medications and glucose-lowering agents given at the discretion of physicians.1
-
AE, adverse event; CI, confidence interval; CV, cardio vascular; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HR, hazard ratio; MG, milligram; P, probability; RRR, relative risk reduction.
PC-IN-103695 Valid till aug 2025
